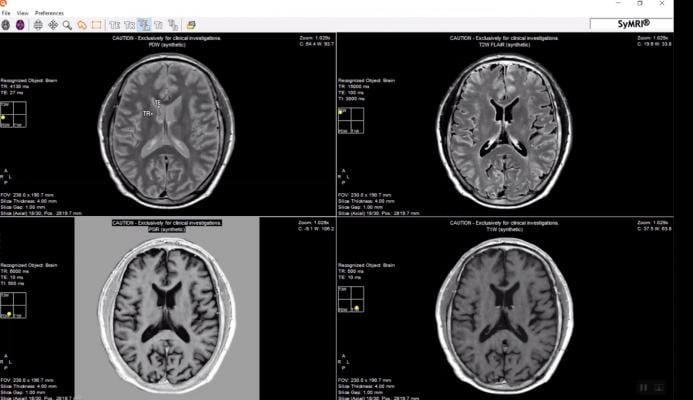
June 14, 2019 — SyntheticMR announced U.S. Food and Drug Administration (FDA) clearance for clinical use of its SyMRI Image and SyMRI Neuro product packages together with magnetic resonance imaging (MRI) systems from Siemens Healthineers.
SyMRI Image is designed to optimize workflow and shorten the exam time using synthetic MRI, where up to eight different contrast images are obtained through an exam of about 6 minutes.
SyMRI Neuro is the next level of quantitative MR, according to the company. The technology measures the absolute tissue properties of the brain and provides quantitative data, automatic segmentation and adjustable contrast images. This provides more information to the clinician and offers objective decision support for diagnosis and follow-up of the patient.
SyMRI is now FDA-cleared for clinical use with MRI systems from GE Healthcare, Philips and Siemens.
For more information: www.syntheticmr.com


 April 24, 2024
April 24, 2024