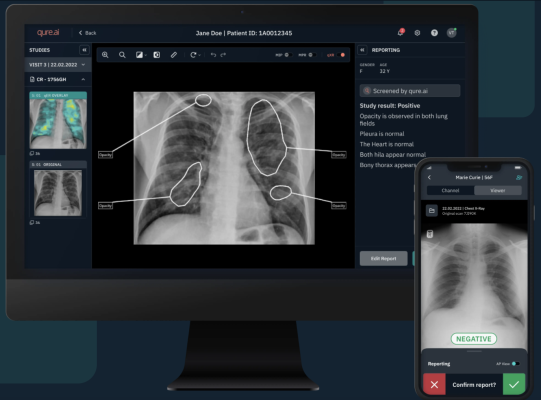
January 8, 2024 — Qure.ai, a leading global innovator in medical imaging solutions, has announced its 13th FDA clearance for their AI-enabled solutions. Qure’s chest X-ray based qXR-LN uses artificial intelligence to identify and localize lung nodules, marking another significant milestone for the organization, strengthening its standing as a pioneer in the realm of AI-powered advancements for plain film radiography and medical imaging. This also marks the 6th FDA clearance for Qure’s chest X-ray based solutions. Notably, this is the only FDA-cleared solution for detecting and localizing lung nodules utilizing computer vision to have Radiologists, Pulmonologists and ER physicians as intended users. The introduction of AI solutions, such as qXR-LN, presents a remarkable opportunity to cast a wider net to identify potentially malignant pulmonary nodules, thereby boosting the fight against lung cancer.
qXR for Lung Nodule (qXR – LN) is a cutting-edge computer-aided detection software designed to identify and highlight regions indicative of suspected pulmonary nodules ranging from 6 to 30 mm in size. Tailored for use in the incidental adult population, this innovative device is a game-changer in diagnostic technology. It can also serve as a crucial second reader for physicians, assisting in the review of frontal (AP/PA) chest radiographs of adults acquired on digital radiographic systems.
Prashant Warier, Co-Founder and CEO of Qure.ai, stated, “With this latest addition to our large series of recent FDA clearances, we are steadfast in our unwavering commitment to the US healthcare space. Having already effectively deployed and implemented this solution globally, this clearance marks yet another ground-breaking leap in our pioneering efforts to combat lung cancer. Our heightened emphasis on the North American marketplace solidifies our commitment to making a meaningful impact in the fight against this deadly disease and underscores our dedication to advancing healthcare through innovation, providing a transformative solution enhancing the early detection of cancer and ultimately improving patient outcomes.”
Qure conducted two pivotal studies to establish the safety and efficacy of its lung nodule device. In the initial pivotal study, Qure successfully demonstrated standalone performance that met predefined success criteria, achieving an Area Under the Curve (AUC) of 94% for nodule detection. The study involved qXR-LN and included chest X-ray scans collected from 8 states and 40 individual sites across the United States. The device's performance was assessed against the ground truth determined by five American board-certified Radiologists. These Radiologists interpreted Chest X-rays alongside corresponding CT scans and reports, with the ground truth based on nodules visible on the Chest X-ray.
In the second pivotal study, a landmark clinical evaluation of qXR-LN was conducted through a multi-reader, multi-case clinical validation study. The performance of various readers, including radiologists, pulmonologists, and emergency room physicians, showed improvement. The qXR-LN algorithm demonstrated a statistically significant and clinically meaningful enhancement in pulmonary nodule detection across all reader groups. Notably, in the multi-reader, multi-case study, the use of qXR-LN resulted in some emergency room physicians and pulmonologists approaching or surpassing the baseline (unaided)performance of radiologists.
As per the American Cancer Society, lung cancer is by far the leading cause of cancer-related deaths in the US, accounting for about 1 in 5 of all cancer deaths[i]. Unfortunately, 44% of cases are not caught until reaching a late stage, when survival rates are less than 8%[ii]; this is where AI-enabled solutions will play a crucial role in reversing this circumstance. Worldwide, 15–20 percent of males with lung cancer are non-smokers, whereas over 50 percent of women with lung cancer are non-smokers[iii]. In the United States alone, about 10% to 20% of lung cancers, or 20,000 to 40,000 cases each year, occur in patients who never smoked or smoked fewer than 100 cigarettes in their lifetime[iv]. This is, unfortunately, a demographic that is often inadvertently overlooked by most cancer screening programs, prolonging the time to diagnosis.
Lung nodule detection on plain film is crucial for the early identification of malignancy risk, enabling timely interventions and improving patient outcomes. Accurate detection aids in treatment planning, disease progression monitoring, and the reduction of false positives and negatives, contributing to cost-efficient and optimal healthcare.
“Incorporating AI algorithms into diagnostic pathways has showcased their transformative impact on the field of medicine. Solutions like Qure.ai's qXR-LN are a significant step towards establishing new possibilities in pulmonary imaging, particularly within oncology. The need for early-stage lung cancer detection is crucial, and tools like qXR-LN can play a significant role in the early detection of incidental nodules", said Dr. Vishisht Mehta, MBBS, FCCP, Director of Interventional Pulmonology, Comprehensive Cancer Centers of Nevada.
Qure.ai's FDA-cleared solutions are reshaping healthcare by introducing cutting-edge innovations across diverse applications. From quantitatively assessing the Cardiothoracic ratio for Cardiomegaly (Heart Failure) to aiding in the placement of Endotracheal Tube and Tracheostomy tube and triaging critical Pneumothorax and Pleural Effusion cases from Chest X-rays, Qure.ai stands at the forefront of diagnostic advancement. The portfolio extends further to triaging acute intracranial hemorrhage cases from non-contrast head CT scans, providing volumetric bleed measurement, cranial fracture detection, and maximal midline shift quantification. Qure.ai continues to redefine healthcare standards through its revolutionary FDA-cleared applications.
Commenting on the announcement, Dr Mannudeep Kalra, Radiologist at Massachusetts General Hospital & Associate Professor of Radiology, Harvard Medical School said, “Integrating AI algorithms in thoracic radiology helps improve radiology workflow and accuracy of interpretation. The results reported in Qure's qXR-LN FDA clearance are similar to our internal evaluation (AUC 0.98; Kaviani et al. Diagnostics 2022). Such results underscore the vital role that AI-driven solutions can play in empowering radiologists to improve patient outcomes. I anticipate that the ongoing evolution of AI technology will significantly advance diagnosis and care.”
The FDA clearance of qXR-LN underscores Qure.ai's ongoing commitment to the American healthcare arena and the unyielding fight against lung cancer. This milestone propels us toward a future where AI-driven technology transforms patient care, playing a pivotal role in elevating radiology and clinical outcomes.
For more information: https://www.qure.ai/

 April 25, 2024
April 25, 2024 








