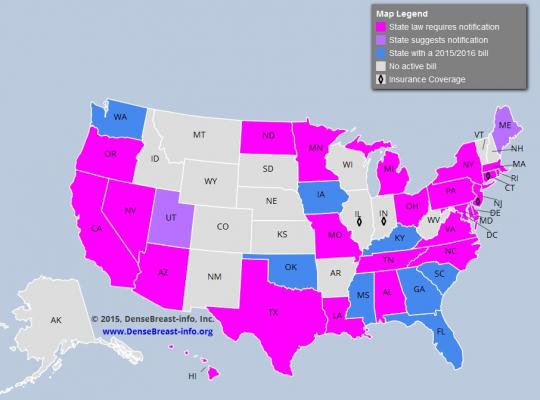
Image courtesy of DenseBreast-info.org
September 25, 2015 — With October and Breast Cancer Awareness Month approaching, educational site www.DenseBreast-info.org reported that 24 U.S. states have adopted legislation requiring healthcare providers to notify women whose mammograms reveal dense fibroglandular breast tissue. To track the progress of legislation across the country, the site has created a color-coded map separating states into five categories:
- States with a law requiring notification on the books (24);
- States that suggest notification (2);
- States with a 2015 or 2016 bill (8);
- States with no active bill (16); and
- States that offer some level of insurance coverage related to breast density screening (4).
Federal legislative efforts are still ongoing, with separate bills introduced in the U.S. House (HR 716) and Senate (S 370); HR 716 had 30 cosponsors as of Sept. 20 and S 370 had 20 cosponsors at that time.
For more information: www.densebreast-info.org

 April 25, 2024
April 25, 2024 








