September 18, 2015 — Edinburgh Molecular Imaging Ltd. (EM Imaging) has signed an exclusive global license for a novel optical imaging agent that could improve the detection of early-stage colorectal cancer.
EM Imaging recently signed the licensing agreement with GE Healthcare Ltd. The company will now complete the development of the imaging agent “EMI-137” that can help doctors identify colorectal cancer early.
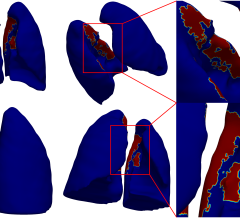
In a recent scientific study, reported in the scientific journal Nature Medicine, the EMI-137 agent allowed doctors to see more early-stage colorectal cancer (CRC) and precancerous tumors, which can then easily be removed via colonoscopy. However, screening with a colonoscope, which is currently the most common method, can miss up to 25 percent of precancerous growths, especially smaller, flat lesions.
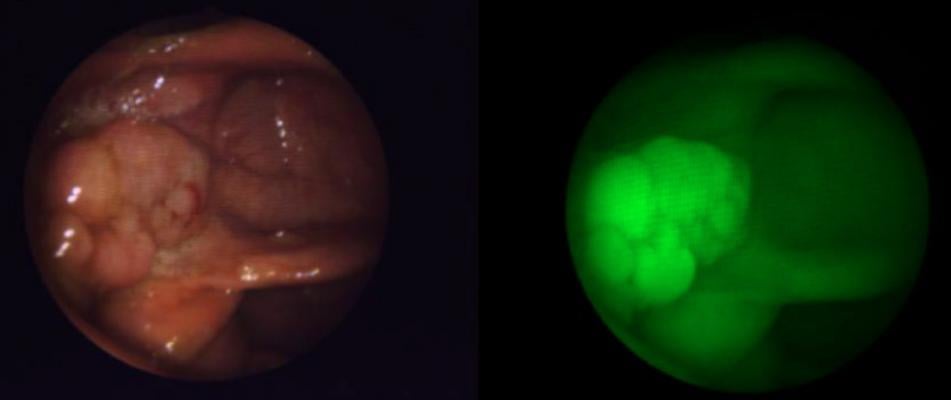
James Hardwick, M.D., lead investigator on the study said, “Of the 47 precancerous polyps detected in this study, 12 were missed using a standard colonoscope. This underlines how unreliable this method can be, and we therefore welcome life-saving new technology like EMI-137. This agent has the potential to make polyps light up like light bulbs – allowing clinicians to detect and remove more polyps, prevent more cancers and save more lives.”
Evidence that colorectal cancer can be prevented by the removal of pre-cancerous lesions and polyps (abnormal growth of tissue) is strong. The EMI-137 agent can help doctors more easily identify these suspicious lesions, take a sample (biopsy) or remove the lesion completely. Colorectal cancer is the second most common cause of cancer in women and the third most common in men, and is a major cause of death.
The Phase I/IIa study in 35 subjects (20 healthy volunteers and 15 patients with high risk of CRC) showed that optical molecular imaging using the fluorescent agent is feasible and safe.
Fluorescence colonoscopy in patients receiving intravenous EMI-137 enabled the visualization of all neoplastic polyps that were visible with white light, and additionally, detected previously missed polyps that were not visible with white light alone. This enables the detection of polyps missed by other techniques.
EMI-137 is a water-soluble compound consisting of a 26–amino acid cyclic peptide, conjugated to a fluorescent cyanine dye, that binds to human tyrosine kinase (c-Met), a receptor frequently overexpressed during cancer growth. EMI-137 has the potential on intravenous administration to image a wide range of cancers — including breast, esophageal, ovarian, thyroid, bile duct carcinoma and lung cancer — due to its specific targeting of the c-Met–receptor.
Sian Godwin, head of licensing at GE Healthcare, said, “We’re pleased EM Imaging will develop and commercialize GE-137, as optical agents can be useful in clinical and research settings for a wide range of common diseases. We see broad partnership and licensing across our research and development portfolio as a way to increase access to new technologies in precision medicine, help develop better diagnostics for patients and reduce the cost of healthcare.”
Neville Young, M.D., program manager, Colorectal Therapies Healthcare Technologies Cooperative, said, "The NIHR [National Institute for Health Research]-funded Colorectal Therapies HTC, based at the University of Leeds, drives a national network of clinicians and academics who are funded to support the development of innovative new technologies that have the potential to benefit patients affected by colorectal disease. We are excited to be working with EM Imaging to help demonstrate both the cost effectiveness and clinical efficacy of their new colorectal tumo r tagging peptide. This technology offers the possibility to identify and remove more easily any cancerous polyps in a patient undergoing a colonoscopy.”
For more information: www.edinimage.com

 December 01, 2025
December 01, 2025