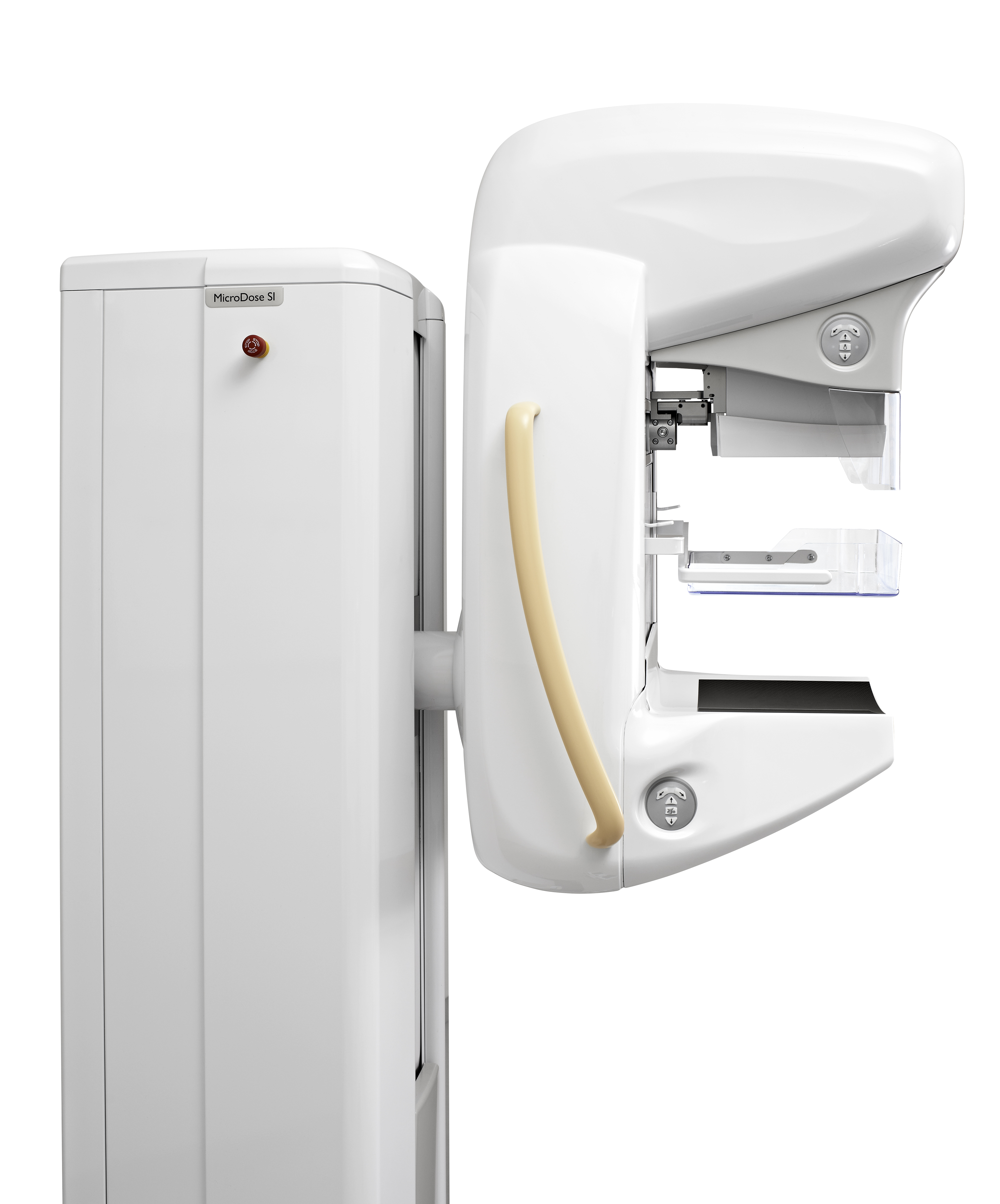
Royal Philips Electronics announced it received 510(k) clearance from the Food and Drug Administration (FDA) for its MicroDose SI system, the first full-field digital mammography (FFDM) system on the market with the capability to enable future Single-Shot Spectral Imaging applications*. Philips is working on future software applications like Spectral Breast Density Measurement*, which will build upon the MicroDose SI technology.
High breast density is a known risk factor for breast cancer — women with high breast density (as seen on a mammogram) are four to five times more likely to get breast cancer than women with low breast density[1]. Additionally, high breast density features a high proportion of connective tissue, which blocks X-rays, making it difficult for clinicians to interpret breast images. As a result, categorization of breast density has become mandatory in many countries and several states in the United States. Unfortunately, there is not yet a standardized method for assessing breast density, which has limited making use of the density categorization for clinical decisions. The most frequently used method of breast density assessment is subjective manual and visual inspection of the image — different radiologists may give different scoring of breast density for the same image.
“Philips believes that spectral imaging technology will be important in helping clinicians to assess breast density and provide personalized care to women,” said Lakshmi Gudapakkam, senior vice president and general manager of diagnostic X-ray and mammography solutions, Philips Healthcare. “With the MicroDose SI, Philips contributes to breast cancer screening by delivering the same low dose, high image quality and ergonomics it already offers, while supplying clinicians with spectral-ready technology.”
As in existing Philips MicroDose systems, MicroDose SI uses unique digital photon-counting technology, which represents a paradigm shift in mammography by enabling clinicians to conduct exams using low radiation dose without compromising image quality. Philips has further advanced this unique technology with Single-Shot Spectral Imaging, which is built upon the fact that breast density is subject to different tissue types and materials that absorb X-rays at various energies. The technology powering the MicroDose SI uses this fundamental behavior of X-rays, allowing clinicians to see more than just a shadow in mammogram images by separating high and low energy X-ray within one single exposure.
“The Philips MicroDose SI technology shows great potential,” said Etta Pisano, M.D., dean of the college of medicine at the Medical University of South Carolina (MUSC). “I look forward to getting the unit installed at MUSC.”
Key advantages of MicroDose SI include:
• High image quality at low X-ray dose (18 to 50 percent lower radiation dose than other digital mammography systems[2],[3],[4],[5], with an average dose reduction of 40 percent**)
• Short exam time - less than 5 minutes including image acquisition
• Patient comfort with anatomically curved and warmed breast support
• Ready for future Single-Shot Spectral Imaging applications*
*Not available in North America
** The actual result of the average dose reduction will vary based on variations in digital mammography systems
For more information: www.healthcare.philips.com
[1] Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 356(3):227-36, 2007.
2 Oduko, J.M. Young, K.C., Burch, A.,: A Survey of Patient Doses from Digital Mammography Systems in the UK in 2007 to 2009. Digital Mammogr. IWDM 2010, 365–370, (2010).
3 Baldelli P., et. al., COMPREHENSIVE DOSE SURVEY OF BREAST SCREENING IN IRELAND, Radiation Protection Dosimetry , Vol. 145, No. 1, pp. 52–60, (2010).
4 Leitz W, Almén A. Patientdoser från röntgenundersökningar i Sverige – utveckling från 2005 till 2008. SSM 2010-14, ISSN 2000-0456, available online (in Swedish) at www.stralsakerhetsmyndigheten.
5 White paper, Comparison of Dose Levels in a National Mammography Screening Program, Philips Healthcare
[1] Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 356(3):227-36, 2007. [2] Oduko, J.M. Young, K.C., Burch, A.,: A Survey of Patient Doses from Digital Mammography Systems in the UK in 2007 to 2009. Digital Mammogr. IWDM 2010, 365–370, (2010). [3] Baldelli P., et. al., COMPREHENSIVE DOSE SURVEY OF BREAST SCREENING IN IRELAND, Radiation Protection Dosimetry , Vol. 145, No. 1, pp. 52–60, (2010). [4] Leitz W, Almén A. Patientdoser från röntgenundersökningar i Sverige – utveckling från 2005 till 2008. SSM 2010-14, ISSN 2000-0456, available online (in Swedish) at www.stralsakerhetsmyndigheten. [5] White paper, Comparison of Dose Levels in a National Mammography Screening Program, Philips Healthcare


 April 16, 2024
April 16, 2024