February 11, 2015 — Sanovas Inc. announced that one of its clinical advisers is warning that the United States healthcare system faces a shortage of therapeutic resources as Medicare coverage of lung cancer screening for at-risk beneficiaries goes into effect. Lung cancer is the leading cause of cancer death and the second-leading cause of all deaths in the United States.
"The U.S. healthcare system is about to be deluged with diagnoses of early-stage lung cancers, and therefore we need to develop appropriate therapies for peripheral tumors, where the majority of lung cancers begin," said Gordon H. Downie, M.D., Ph.D., FCCP whose clinical research has been published in peer-reviewed medical journals such as American Journal of Respiratory Critical Care Medicine,CHEST, and Journal of Bronchology. Downie is a pulmonologist at Northeast Texas Interventional Medicine and a clinical adviser to Sanovas.
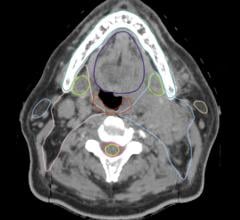
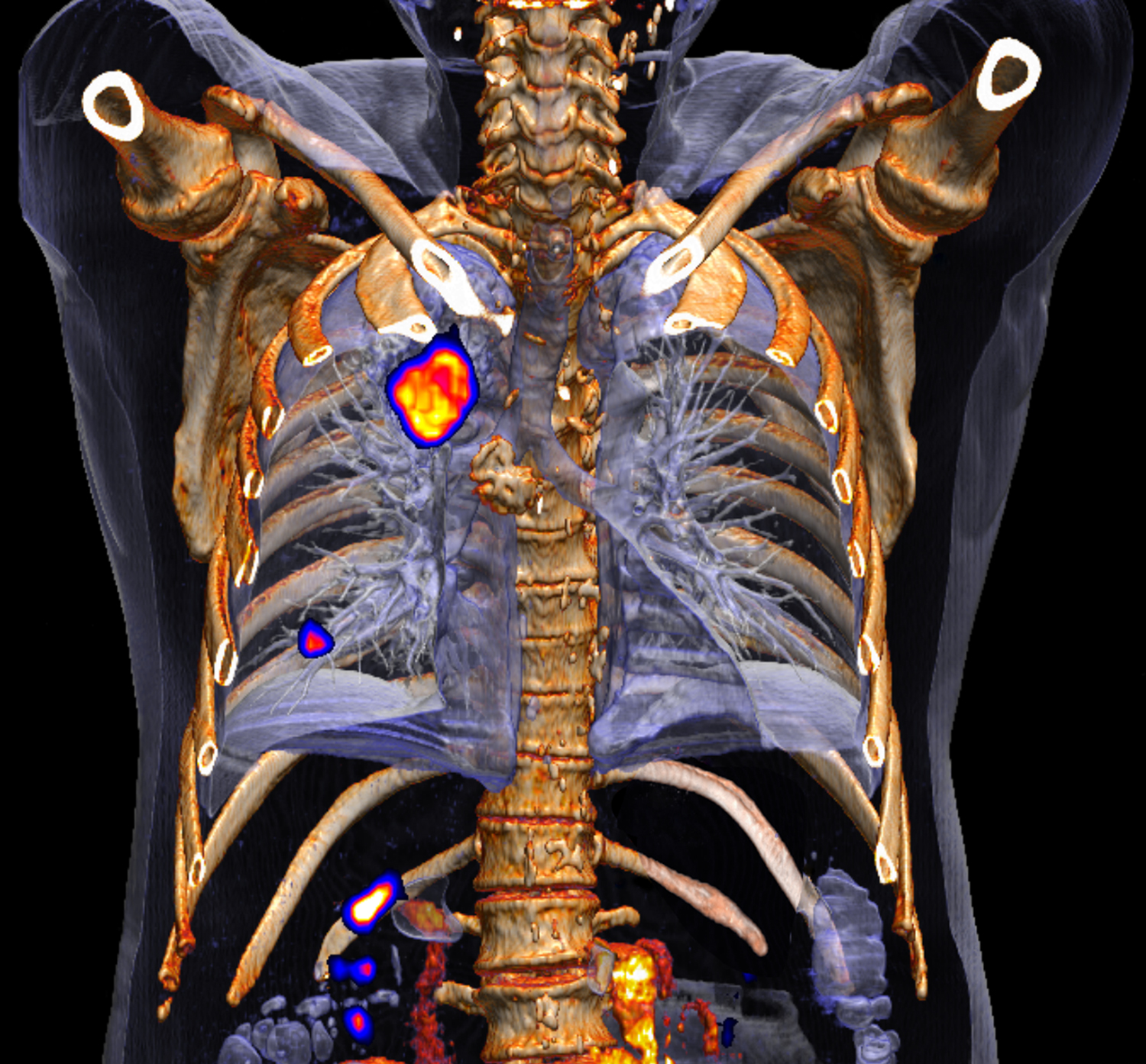
"Medicare coverage has a 'good news/bad news' scenario to it. On the one hand, screening at-risk Medicare beneficiaries with CT (computed tomography) scans will absolutely reduce the number of deaths from lung cancer. On the other hand, our country's healthcare system is going to be inundated with patients who have been diagnosed with early-stage lung cancer, a potential order-of-magnitude spike in the diagnosis of peripheral nodules," explained Downie. "This expected significant increase in the diagnosis of early stage lung cancers will stress the resources now available for treating early stage lung cancers."
Results of the National Lung Screening Trial (NLST), funded by the National Cancer Institute (NCI) and published in the August 2011 edition of the New England Journal of Medicine, concluded that "screening with the use of low-dose CT reduces mortality from lung cancer." Subsequently, in November 2014, the U.S. Centers for Medicare & Medicaid Services (CMS) announced that, beginning in 2015, Medicare coverage of low-dose CT lung cancer screening for at-risk beneficiaries will be required by law. Approximately 4 million Medicare beneficiaries fit the eligibility criteria for screening, according to the Lung Cancer Alliance (LCA).
"What are we going to find? A boatload more cancers, but in the peripheral airways of the lungs, not in the central airways. How do we want to deal with them? That's the question," said Downie. "Surgery and radiation for treatment of peripheral early stage lung cancer are current standards but there are excellent alternative treatments and these certainly need to be explored. But how do you get these alternative treatments to these hard to reach peripheral locations in the lung? Of course, you need small-diameter technologies to get to the periphery of the lung and then, once you get out there, you've got to be able to deliver something locally and focally. What is that solution? Photodynamic therapy, or PDT. PDT can be delivered locally and focally without having to systemically toxify the patient for a small centimeter nodule that's out in the periphery of the lungs and is starting to metastasize.
"One of the most common problems encountered during radiation therapy of malignant tumors is that the tumor cells become deficient in oxygen - a condition referred to as hypoxia," explained Downie. "It looks promising that the Sanovas PDT technology addresses the hypoxia dilemma. As a long-time proponent of using PDT technology to address peripheral lung tumors, I am truly excited by the potential this approach could lead to, and I look forward to seeing clinical research to confirm the promise this patent suggests."
For more information: www.sanovas.com


 April 23, 2024
April 23, 2024