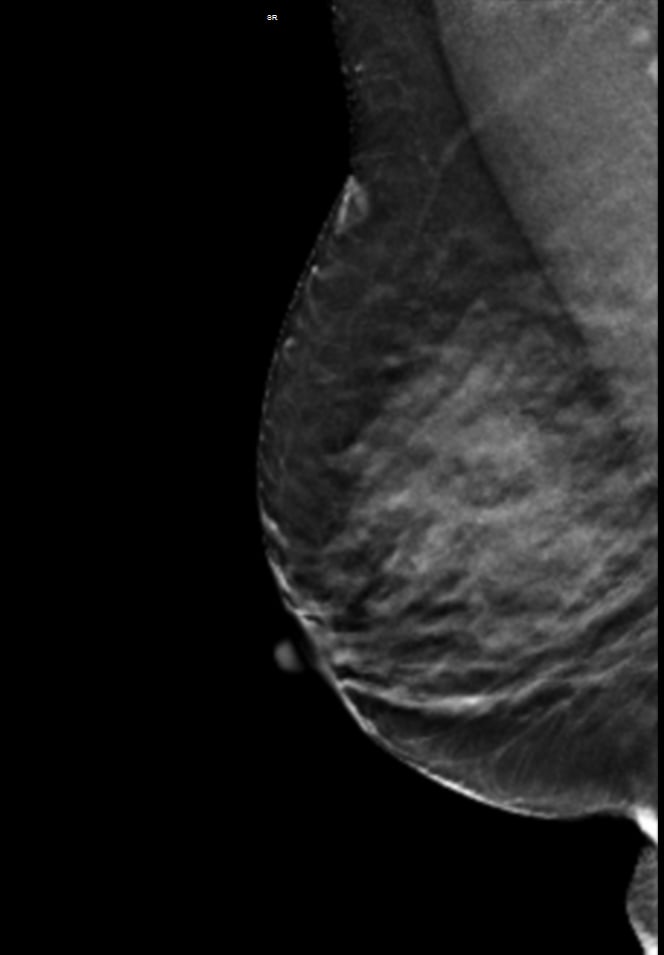
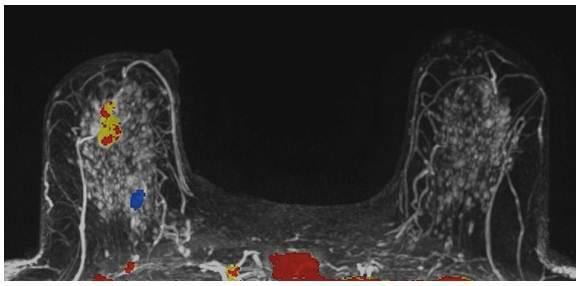
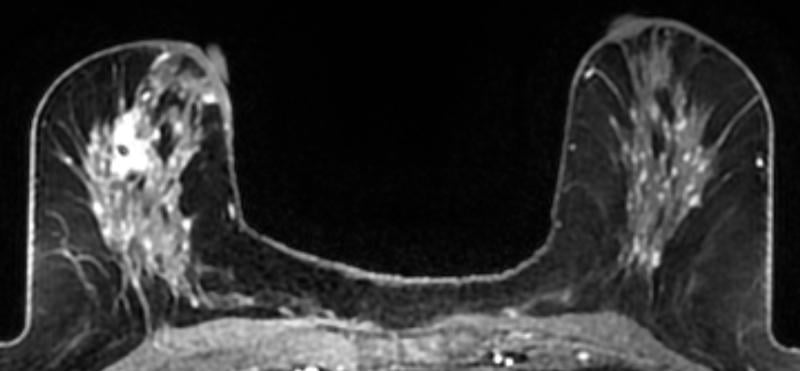
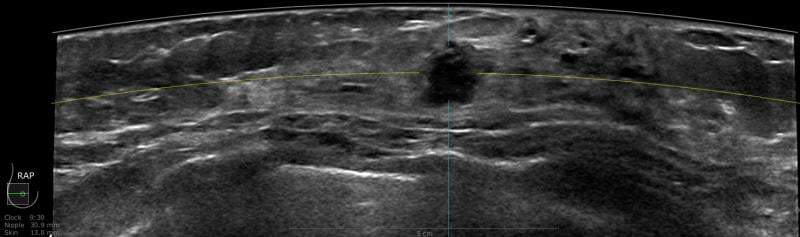
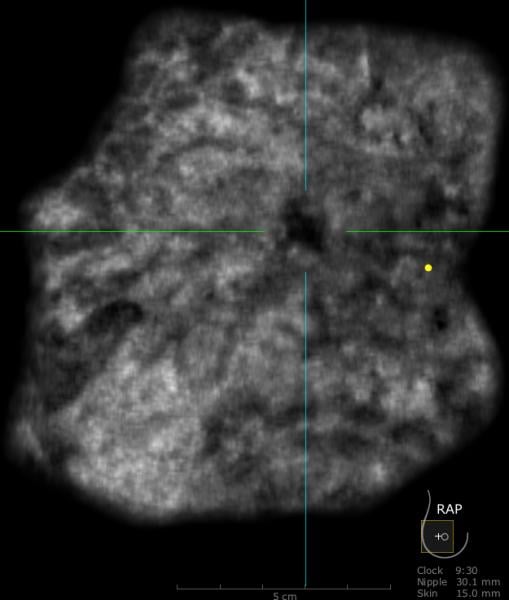
A 59-year-old female presented with a 3 cm palpable mass in the right breast. No identifiable abnormalities were identified on either the RMLO (on right) or RCC (on left) tomosynthesis images. (Image courtesy of Marc F. Inciardi, M.D., University of Kansas Medical Center)
Since the implementation of routine screening mammography, we have seen a 30 percent reduction in breast cancer mortality. Despite these results, mammography remains at the center of controversy, with seemingly conflicting studies on its effectiveness published on a regular basis.
While mammography remains the gold standard for detecting breast cancer, research has shown that it is not equally effective in all women. In women with dense breast tissue, mammography can miss between 37 and 70 percent of breast cancers.1-4 This leads to a delay in diagnosis and a worse prognosis for women with dense breast tissue.5,6 In addition to having dense breast tissue mask cancers, women who have dense breast tissue also have a four- to six-times elevated risk of developing breast cancer.7,8 Lastly, Yaghjyan et al. reported that the neoplastic features found in women with dense breast tissue are more aggressive.9
Recent advances in technology, particularly the introduction of full field digital mammography (FFDM), have improved the effectiveness of mammography. The Digital Mammographic Imaging Screening Trial (DMIST) found that digital mammography performed better than screen-film mammography (SFM) in certain subgroups, including women with dense breast tissue.10 However, a more recent study by Hoff et al. reports no difference in the number of missed cancers when comparing FFDM to SFM.11 Additionally, a large-scale trial found that digital mammography was less than 60 percent sensitive in women who have dense breast tissue vs. 98 percent sensitive in women with fatty breast tissue.10
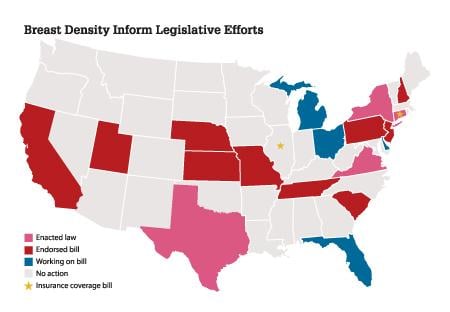
Ongoing research on the impact of breast density on mammography’s sensitivity, combined with national attention on the growing number of “density inform” legislative efforts (see the map at right) have increased awareness of mammography’s limitations, both to the imaging community and the general public. This has heightened attention on the need for supplemental imaging to reduce missed cancers, with minimal impact on false positives and anxiety.
Clearly, supplementing mammography with advanced imaging should be considered for women with dense breast tissue. Unfortunately, clinicians are being asked to navigate through an array of supplemental imaging without guidance on best practices.
This article will review the current literature and develop an evidence-based approach to supplemental imaging for women with dense breast tissue by concentrating on two U.S. Food and Drug Administration (FDA)-approved supplemental techniques, digital breast tomosynthesis (DBT) and automated breast ultrasound (ABUS).
Tomosynthesis
One of the issues with conventional mammography that impacts its accuracy in cancer detection stems from the overlap of breast structures, which creates shadows that can be read as false positives (FPs). DBT helps eliminate the overlap problem and has been shown to reduce false positives by 30 to 39 percent.12,13 For a DBT exam, the breast is positioned and compressed in the same way as a mammogram. DBT produces a 3-D image of the breast by using several low-dose X-rays obtained at multiple angles. Objects at varying depths in the breast are projected at different angles, and reconstruction leads to a series of slices designed to distinguish each layer. DBT also improves the characterization of masses.14-17 Currently, only one vendor, Hologic, has received FDA approval for its Selenia Dimensions tomosynthesis X-ray mammography system for use as an adjunct to standard 2-D mammography. The highest rate of false positives is seen in young women with dense breast tissue.18 Therefore, screening these women with DBT would be effective in reducing recall rates.
While the reduction of false positives with DBT is well documented,13 sensitivity in dense breast tissue is still lagging. A number of studies have demonstrated comparable sensitivity between DBT plus FFDM and FFDM alone.12,19,20,21 The Hologic 2011 pre-market approval (PMA) reports only a 10.7 percent sensitivity gain in the pivotal reader study 1. At the 2011 annual Radiological Society of North America (RSNA) event, Rafferty presented data demonstrating that the addition of DBT to FFDM allows for an increase in cancer detection of 9.7 percent in women with dense breast tissue.
These studies demonstrate conflicting and variable results for the sensitivity of DBT when compared to FFDM. Additionally, changes in specificity vary substantially. Even with the advancements of DBT, X-ray mammography still has inherent limitations in women with dense breast tissue.
There is also the issue of radiation dose. The dose for FFDM-DBT is twice FFDM alone.22-24 Although the combined dose remains under the limit set by the FDA Mammography Quality Standards Act (MQSA), the cumulative effect of DBT’s increased dose is unknown, especially for an annual screening exam. Prior reports have speculated that one in a thousand women who have routine screening mammograms will develop radiation-induced breast cancer.25 If the dose is doubled, how does that translate into risk?
Having only one DBT view would minimize dose, but may result in a significant reduction in the sensitivity of DBT.36 The As Low As Reasonably Achievable (ALARA) rule highlights the importance of radiation awareness. Digital mammography is 98 percent effective in women with fatty breast tissue.10 Therefore, not every patient will benefit from the doubled dose of FFDM-DBT. For women with dense breast tissue, the adjunct of screening ultrasound offers a non-ionizing alternative.
Automated Breast Ultrasound
Ultrasound is a proven diagnostic tool in breast imaging; however, the limitations of traditional hand-held ultrasound (HHUS), which include operator-dependent variability and elevated acquisition times, make it unwieldy for broad-scale screening. These limitations are largely overcome by automated breast ultrasound (ABUS) imaging.26
The somo·v ABUS was recently approved by the FDA as a supplemental screening tool. The somo·v ABUS technology, much like HHUS, uses high-frequency sound waves that reflect at boundaries between tissues and structures within the breast. However, ABUS allows for automated 3-D ultrasound imaging of the whole breast.
The sensitivity benefit of using whole breast ultrasound to screen women with dense breast tissue is well documented.27-31 Mammography and ultrasound together have been shown to be up to 97 percent sensitive.1 Kelly et al. showed that supplementing mammography with ABUS resulted in doubling the cancer detection rate.32 The ACRIN 6666 trial showed that supplementing mammography with screening ultrasound in women with an elevated risk of developing breast cancer and dense breast tissue increased detection rate by 55 percent.
Hooley et al. recently reported in Radiology an additional cancer detection yield of 3.2/1,000 for screening breast ultrasound. This coincides with prior studies demonstrating between a 2.7-4.6/1,000 yield. More than 90 percent of the mammographically occult malignant lesions detected with ultrasound are invasive, and the majority are early, node-negative cancers.3,28,33 As a result, ultrasound continues to be an invaluable adjunct to mammography for the detection of breast cancer.
The benefits of screening ultrasound were highlighted in U-Systems’ PMA Application. This included data from a multi-reader, multi-case receiver operating characteristic (ROC) study that demonstrated a 31 percent improvement in sensitivity and minimal change in specificity with ABUS plus FFDM when compared to FFDM alone.34 This contrasts the minimal improvement in sensitivity described with DBT.
Additionally, when comparing shifts in the ROC curves, there is a higher percent increase in the area under the curve (AUC) with ABUS as opposed to DBT. DBT was found to have a 7 percent increase in the area under the ROC curve for the intended use population,35 whereas the ABUS reader study demonstrated a 14 percent improvement in performance for the intended use population.30
An Evidence-Based Approach
While DBT reduces false positives, it does not offer the sensitivity advantage of ABUS. Given the large discrepancy in cancer detection rates for women with dense breast vs. fatty breast tissue, a combination approach is advised. These two tests can complement each other to optimize cancer detection and potentially improve positive predictive value (PPV).
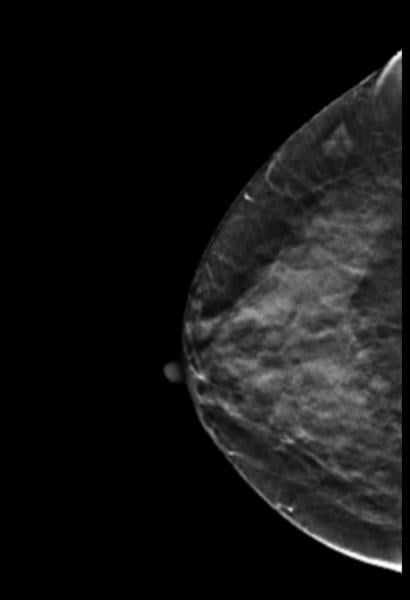
Why perform different exams? By virtue of physics and tissue characteristics, imaging techniques may significantly differ in the characteristics of cancers detected. This can be seen in everyday practice with mammography, ultrasound and MRI of the breast. In fact, Hoff et al. demonstrated differences in the discernible features of cancer by comparing two similar modalities, FFDM and SFM.11 Using different supplemental tools to complement each other will result in improved sensitivity and specificity (see the images of a patient case on page 47 for an example).
An example of this can be seen with segmental or clustered microcalcifications. Some studies have shown that microcalcifications are better detected on FFDM than DBT.31,36 Ultrasound and magnetic resonance imaging (MRI) do not routinely detect microcalcifications but may detect associated masses not visible on mammography. Using the various detection tools available, a combined approach can further characterize findings and improve sensitivity.
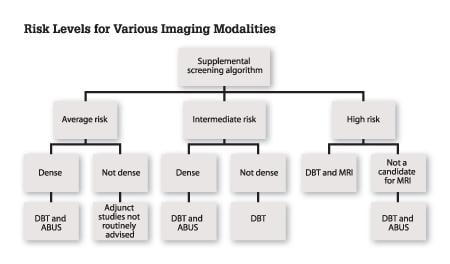
As the healthcare system moves closer to achieving personalized medicine, there is clearly a need for individualizing breast care. One size does not fit all, as evidenced by the limitations of mammography. An evidence-based approach to navigating through the FDA-approved supplemental imaging tools available for screening mammography is diagrammed in Figure 1.
Since not all women have the same risk of developing breast cancer, prior to determining a breast health plan, an assessment of individual risk should be performed. If a patient is deemed to be at high risk, then a more robust pathway and education on surveillance and prophylactic intervention could be offered. In women with moderate or average risk, further determination of the patient’s breast density must be performed to identify the appropriate combination of imaging. Each imaging tool should be specifically used for its proven benefits. Examples of using an evidence-based approach include:
• using DBT in a diagnostic setting to better characterize lesions14,15,16,17
• avoiding the use of screening breast MRI in average-risk women due to the elevated false positive rate37,38
• using ABUS as a screening tool for women with dense breast tissue to improve sensitivity
Cost and workflow issues must also be considered. DBT doubles the read time of FFDM,39 which had already increased when compared to SFM.40 ABUS increases the read time by approximately 3 minutes.41 Although the coupled approach for evaluating dense breasts may increase the total screening time, the reduction of false positives, decrease in unnecessary workups and biopsies, and the benefit of finding cancers earlier will offset the increased read times and cost of the supplemental exams.
A Combined Approach — Best for Women With Dense Breasts
For many women, mammography is an effective tool for the detection of breast cancer. However, research has demonstrated that for women with dense breast tissue, supplemental imaging is necessary to reduce the number of missed cancers.
The use of DBT may reduce false positives, and there is a significant breast cancer detection benefit when using screening ultrasound to supplement annual mammography in women with dense breast tissue. As a result, automated breast ultrasound coupled with digital breast tomosynthesis provides optimization of sensitivity while reducing false positives. This combined approach is the most advantageous screening choice for the majority of women with dense breast tissue. itn
Additional Resources on ITN for Breast Imaging and Dense Breast technology Information
VIDEO: Advances and Trends in Breast Imaging
Mammographic Breast Density — What It Means
Breast Density: Are You Informed?
Jessie Jacob, MD, a board-certified, dedicated breast imager, recently joined U-Systems, a GE Healthcare Company, as Vice President of Medical Affairs. Previously, Dr. Jacob directed breast centers in California and New York and served as Director of Breast Imaging and Intervention at El Camino Hospital in Mountain View, Calif.
References:
1 Kolb TM, Lichy J, Newhouse JH. “Comparison of the performance of screening mammography, physical examination and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations.” Radiology 2002;225:165-75.
2 Mandelson MT et al. “Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers.” J Natl Cancer Inst 2000; 92(13): 1081-1087.
3 Berg WA, Blume JD, Cormack JB, Mendelson EB, Lehrer D, Bohm-Vélez M, Piasano ED, Jong RA, Evans WP, Morton MJ, Mahoney MC, Hovanession Larsen L, Barr RG, Farria DM, Marques HS, Bopari K. “Combined screening with ultrasound and mammography vs. mammography alone in women at elevated risk of breast cancer.” JAMA. 2008;299:2151–2163.
4 Carney PA, Miglioretti DL, Yankaskas BC, et al. “Individual and combined effects of age, breast density and hormone replacement therapy use on the accuracy of screening mammography.” Ann Intern Med 2003;138:168–175.
5 Roubidoux MA, Bailey JE, Wray LA, Helvie MA. “Invasive cancers detected after breast cancer screening yielded a negative result: relationship of mammographic density to tumor prognostic factors.” Radiology 2004 Jan;230:42-48.
6 Sala E, Solomon L, Warren R, et al. “Size, node status and grade of breast tumours: association with mammographic parenchymal patterns.” Eur Radiol 2000;10:157–61.
7 McCormack VA, dos Santos Silva I. “Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis.” Cancer Epidemiol Biomarkers Prev 2006;15:1159–1169.
8 Boyd NF, Guo H, Martin LJ, et al. “Mammographic density and the risk and detection of breast cancer.” N Engl J Med 2007;356:227–236.
9 Lusine Yaghjyan, Graham A. Colditz, Laura C. Collins, Stuart J. Schnitt, Bernard Rosner, Celine Vachon and Rulla M. Tamimi. “Mammographic Breast Density and Subsequent Risk of Breast Cancer in 7 Postmenopausal Women According to Tumor Characteristics.” J Natl Cancer Inst (2011) 103 (15): 1179-1189.
10 Pisano ED, Hendrick RE, Yaffe MJ, et al. “Diagnostic accuracy of digital versus film mammography: exploratory analysis of selected population subgroups in DMIST.” Radiology 2008;246:376–383.
11 Hoff SR, Abrahamsen AL, Samset JH, et al. “Breast Cancer: Missed Interval and Screening-Detected Cancer at Full-Field Digital Mammography-Results from a Retrospective Review.” Radiology June 14, 2012; e-published before print.
12 Gur D, Abrams GS, Chough DM., et al. “Digital Breast Tomosynthesis: Observer Performance Study.” AJR 2009; 193(2): 586-591.
13 Rafferty EA, Niklason L, Halpern E et al. “Assessing Radiologist Performance Using Combined Full-Field Digital Mammography and Breast Tomosynthesis Versus Full-Field Digital Mammography Alone: Results of a Multi-Center, Multi-Reader Trial.” Presented at RSNA 2007, Session SSE26-02 Late Breaking Multicenter Clinical Trials.
14 Fornvik D, Zackrisson S, Ljunberg O, et al. “Breast Tomosynthesis: Accuracy of tumor measurement compared with digital mammography and ultrasonography.” Acta Radiol 2010; 3: 240-247.
15 Michell M, et al. “Digital Breast Tomosynthesis: A Comparison of the Accuracy of Digital Breast Tomosynthesis, Two-Dimensional Digital Mammography and Two-Dimensional Screening Mammography (Film-Screen).” Breast Cancer Research 2009, 11(Suppl 2):01.
16 Meacock LM, et al. “The accuracy of breast cancer size measurement: Digital breast tomosynthesis (DBT0 vs. 2D digital mammography (DM).” European College of Radiology annual meeting. Vienna, Austria, 2010.
17 Tagliafico A, Astengo D, Cavagnetto F, et al. “One-to-one comparison between digital spot compression view and digital breast tomosynthesis.” Eur Radiol 2012 Mar;22(3):539-44.
18 Elmore JG, Barton MB, Moceri VM, Polk S, Arena PJ and Fletcher SW. “Ten-year risk of false positive screening mammograms and clinical breast exams.” NEJM. 1999;338:1089–96.
19 Zuley M, Sumkin J, Ganott M, Bandos A, Kelly A, Lu A, Catullo V, Chough D, Gur D, Perrin R. “Digital Breast Tomosynthesis vs. Supplemental Diagnostic Mammography Images for the Evaluation of Noncalcified Breast Lesions.” Presented at RSNA 2011, MSVB31-06 Breast Series: Emerging Technologies in Breast Imaging.
20 Gennaro G, Toledano A, di Maggio C, Baldan E, Bezzon E, La Grassa M, Pescarini L, Polico I, Proietti A, Toffoli A, Muzzio PC. “Digital breast tomosynthesis versus digital mammography: A clinical performance study.” Eur Radiol. 2010 Jul;20(7):1545-53.
21 Teertstra HJ, Loo CE, van den Bosch MA, van Tinteren H, Rutgers EJ, Muller SH, Gilhuijs KG. “Breast tomosynthesis in clinical practice: Initial results.” Eur Radiol. 2010 Jan;20(1):16-24.
22 Executive Summary for FDA Advisory Panel available online at: www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Med…
23 Baker JA, Lo JY. “Breast tomosynthesis: State-of-the-art and review of the literature.” Acad Radiol. 2011;18:1298-1310.
24 Dobbins JT III. “Tomosynthesis imaging: at a translational crossroads.” Med Phys. 2009;36:1956-1967.
25 Yaffe MJ, Mainprize JG. “Risk of radiation-induced breast cancer from mammographic screening.” Radiology. 2011 Jan;258(1):98-105.
26 Lin X, Wang J, Han F, et al. “Analysis of eighty-one cases with breast lesions using automated breast volume scanner and comparison with handheld ultrasound.” Eur J of Radiol. 2012 May;81(5):873-8.
27 Kolb TM, Lichy J, Newhouse JH. “Occult cancer in women with dense breasts: Detection with Screening US — Diagnostic Yield and Tumor Characteristics.” Radiology 1998 Apr 207:191-9.
28 Leconte I, et al. “Mammography and Subsequent Whole-Breast Sonography of Nonpalpable Breast Cancers: The Importance of Radiologic Breast Density.” AJR. 2003 Jun;180:1675-1679.
29 Kaplan SS. “Clinical utility of bilateral whole-breast US in the evaluation of women with dense breast tissue.” Radiology 2001;221:641-64.
30 Harvey JA, Bovbjerg VE. “Quantitative assessment of mammographic breast density: relationship with breast cancer risk.” Radiology 2004;230: 29-41.
31 Buchberger W, Niehoff A, Obrist P, DeKoekkoek-Doll P, Dunser M. “Clinically and mammographically occult breast lesions: detection and classification with high-resolution sonography.” Semin Ultrasound CT MR 2000;21:325-36.
32 Kelly KM, Richwald GA. “Automated Whole Breast Ultrasound: Advancing the Performance of Breast Cancer Screening.” Semin Ultrasound CT MRI 2011; 32:273-280.
33 Crystal P, Strano SD, Shcharynski S, Koretz MJ. “Using sonography to screen women with mammographically dense breasts.” AJR Am J Roentgenol 2003;181:177-82.
34 U-systems PMA P110006 FDA’s Summary of Safety and Effectiveness Data
35 Hologic PMA P080003 — FDA’s Summary of Safety and Effectiveness Data
36 Poplack SP, Tosteson TD, Kogel CA, et al. “Digital breast tomosynthesis initial experience in 98 women with abnormal digital screening mammography.” Am J Roentgenol. 2007;189:616-623.
37 Lawrence WF, Liang W, Mandelblatt JS, et al. “Serendipity in diagnostic imaging: magnetic resonance imaging of the breast.” J Natl Cancer Inst 1998 Dec 2;90(23):1792-800.
38 Kuhl CK, Bieling HB, Gieseke J, et al. “Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclical-phase dependency.” Radiology 1997;203 (1):137-44.
39 Skaane P, Gullien R, Eben E, Ekseth U, Haakenaasen U, Jahr G, Jebsen I, Krager M. “Reading Time of FFDM and Tomosynthesis in a Population-based Screening Program.” Presented at RSNA 2011, MSVB31-07 Breast Series: Emerging Technologies in Breast Imaging.
40 Berns EA, Hendrick RE, Solari M, Barke L, Reddy D, Wolfman J, Segal L, DeLeon P, Benjamin S, Willis L. “Digital and screen-film mammography: comparison of image acquisition and interpretation times.” Am J Roentgenol. 2006 Jul;187(1):38-41.
41 Brem RF, Rapelyea JM, Torrente J, et al. “Radiologist Interpretation Time for 3D Automated Breast Ultrasound Screening.” Presented at the 2012 American Roentgen Ray Society annual meeting in Vancouver, Canada.


 December 08, 2025
December 08, 2025