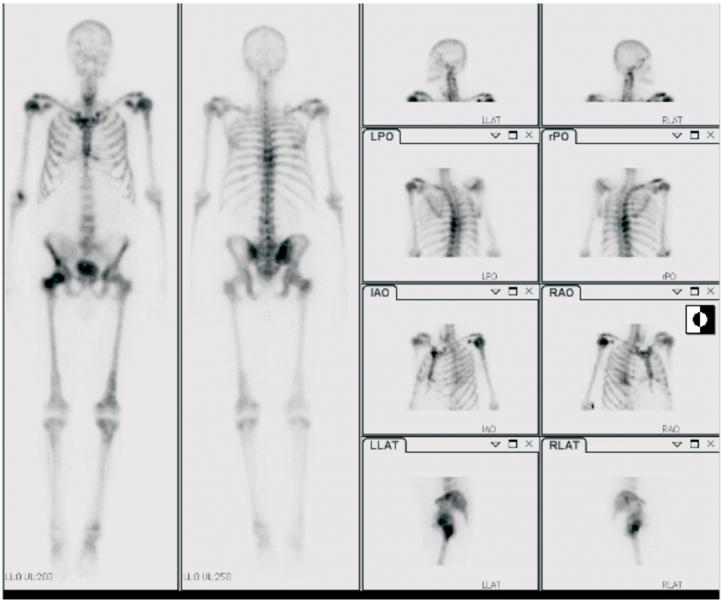
Whole-body 131I imaging in a 52-year-old woman with papillary thyroid cancer post thyroidectomy. Radiotracer uptake in the neck is seen at sites of residual malignant disease. 131I was subsequently given to ablate the residual sites of malignant disease.
There has been growing discussion and excitement surrounding the possibility of personalized medicine and targeted radionuclide therapy (TRT) in recent years.1 TRT refers to the use of one or more radionuclides that may be incorporated into a conjugate or attached to a ligand for targeted therapy at the cellular or molecular level. Radioimmunotherapy refers to targeted therapy with a radiolabeled monoclonal antibody. TRT has actually been part of routine medical therapy for several years.
Indeed, physicians have been imaging and treating patients with thyroid disease using radioactive iodine since 1946.2 The first use of iodine-131 (I-131) radioimmunotherapy occurred in 1982.3 Theranostics is a process of therapy for individual patients where treatment can be tailored based on imaging results using the same molecule and targets on tumor cells. Using the same or similar radiopharmaceutical for both imaging and therapy provides the opportunity to image disease sites that will be targeted both before and after therapy. It also provides the possibility of seeing what will be treated, estimating the amount of radioactivity that will be taken up at sites of disease versus the amount that will be taken up by normal tissue and calculating the specific amount of radioactivity needed to achieve maximum efficacy with limited toxicity. Figure 1 shows an image of a patient with thyroid disease who was imaged and treated using I-131.
Factors to Consider
There are several factors to consider when using TRT and/or radioimmunotherapy. For example, providing a personalized calculation of radiation exposure and tailoring therapy to ensure optimal treatment with limited toxicity may require several patient visits and may, in the long run, prove challenging both for the treating physician and for the patient.4 There is a need for well-designed multicentered trials comparing TRT and/or radioimmunotherapy with other currently available therapy to generate robust data on patient outcomes such as quality of life and impact on overall survival. The optimal sequencing of therapy including chemotherapy, radiation therapy, TRT and/or radioimmunotherapy remains a topic of debate. The use of imaging to triage patients to receive appropriate therapy, assess the radioactivity dose to be administered and determine treatment effect is under investigation. The need for radiopharmaceuticals to target specific disease types is growing, with accessibility and cost remaining significant concerns.1
These factors have, on occasion, led to barriers limiting the use of TRT and/or radioimmunotherapy in the clinic. For example, the first radioimmunotherapy to receive U.S. Food and Drug Administration (FDA) approval was Y-90-ibritumomab tiuxetan (Zevalin; Spectrum Pharmaceuticals, Inc.), which has been FDA-approved since 2002 but is relatively uncommon in the clinic. Zevalin is a radiolabeled anti-CD20 monoclonal antibody used for the treatment of relapsed or refractory low-grade follicular lymphoma or transformed B-cell non-Hodgkin's lymphoma (NHL), including rituximab-refractory follicular NHL.
According to results in the literature, Zevalin can improve progression-free survival and has been associated with improved overall response rate in advanced follicular lymphoma compared with using rituximab alone.5-7 The main toxicity is cytopenia, with fatigue and nausea being among the most common nonhematologic adverse reactions. The incidence of secondary malignancy is low.7 Further, Zevalin has an advantage in that typically a one-time administration is needed versus multiple cycles of chemotherapy. However, the utilization of Zevalin in recent times has been falling. It is estimated that Zevalin was used to treat 889 patients in 2010 versus 551 in 2013, and slightly less than 500 patients in 2014.1 I-131-tositumomab (Bexxar; GlaxoSmithKline), also a radiolabeled anti-CD20 monoclonal antibody used for the treatment of relapsed or refractory low-grade follicular lymphoma or transformed B-cell NHL, has been FDA-approved since 2003. Bexxar has been shown to provide an overall response rate of 95 percent and complete response, or 75 percent in patients with previously untreated, advanced-stage, low-grade NHL;8 however, Bexxar was withdrawn from the market in 2014 because of low clinical use.
Limitations
The use of Zevalin and Bexxar for the treatment of patients with lymphoma has been limited over the years by several factors. In particular, oncologists have described difficulty finding appropriate sites to refer patients to for radioimmunotherapy. Further, the referral process is complex and can have an impact on continuity of care.1, 4 There is a proliferation of therapy options for patients with cancer and a dearth of definitive up-to-date randomized clinical trial data demonstrating therapy effectiveness and improved outcome. Patients are concerned about radiation safety and education for patients regarding this is limited.1
Several issues have been suggested to improve access to and efficacy of Zevalin in patient care. Perhaps the most important is the need for improving collaboration between members of the healthcare team to promote easy access to and education about the benefits and risks of radioimmunotherapy for both physicians and patients. Easing the method of assessing patient eligibility, post-therapy follow-up and resolving reimbursement concerns with the Affordable Care Act would likely also be helpful.1 Based on our experience with Zevalin, it is hoped the logistical issues related to access and education can be avoided with newer agents.
The first trial of an α emitter in TRT was reported in 1997.9 Although TRT has been used for the palliation of patients with metastatic prostate cancer to bone for several years, Ra-223-dichloride (Xofigo; Bayer Healthcare Pharmaceuticals) was the first radiopharmaceutical shown to extend life in men with castration-resistant disease.10 Indeed, since FDA approval for the use of Xofigo in men with castration-resistant prostate cancer was obtained in 2013, this has become increasingly prevalent in routine oncologic clinical practice as a way to target and treat bone metastases. Figure 2 shows a patient with prostate cancer and widespread bone metastases.
The Future of TRT and Radioimmunotherapy
Today, there is a growing spectrum of TRT and radioimmunotherapy available for the treatment of patients with cancer. For example, in addition to those already discussed, peptide receptor radionuclide therapy (PRRT) in neuroendocrine tumors is showing significant promise. The use of PPRT in patients with metastatic and/or progressive neuroendocrine malignancy is well tolerated and can improve symptomatic control, progression-free survival and overall survival.11, 12
Whether given alone or in combination, it is likely that both TRT and radioimmunotherapy have an important role to play in the treatment of patients with cancer in the years to come.
References
1. Fahey F, Zukotynski K, Capala J, et al. "Targeted radionuclide therapy: proceedings of a joint workshop hosted by the National Cancer Institute and the Society of Nuclear Medicine and Molecular Imaging." J Nucl Med. 2014; 55:337-48.
2. Seidlin SM, Marinelli LD, Oshry E. "Radioactive iodine therapy: effect on functioning metastases of adenocarcinoma of the thyroid." J Am Med Assoc. 1946; 132:838-847.
3. Larson SM, Carrasquillo JA, Krohn KA, et al. "Localization of 131I-labeled p97- specific Fab fragments in human melanoma as a basis for radiotherapy." J Clin Invest. 1983;72:2101-2114.
4. Schaefer NG, Ma J, Huang P, Buchanan J, Wahl RL. "Radioimmunotherapy in non-Hodgkin lymphoma: opinions of U.S. medical oncologists and hematologists." J Nucl Med. 2010;51:987-994.
5. Witzig TE, Gordon LI, Cabanillas F, et al. "Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma." J Clin Oncol. 2002;20:2453-2463.
6. Morschhauser F, Radford J, Van Hoof A, et al. "Phase III trial of consolidation therapy with yttrium-90-ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma." J Clin Oncol. 2008;26:5156-5164.
7. Morschhauser F, Radford J, Van Hoof A, et al. "90Yttrium-ibritumomab tiuxetan consolidation of first remission in advanced-stage follicular non-Hodgkin lymphoma: updated results after a median follow-up of 7.3 years from the international, randomized, phase III first-line indolent trial." J Clin Oncol. 2013;31:1977-1983.
8. Kaminski MS, Tuck M, Estes J, et al. "131I-tositumomab therapy as initial treatment for follicular lymphoma." N Engl J Med. 2005;352:441-449.
9. Jurcic JG, McDevitt MR, Sgouros G, et al. "Targeted alpha-particle therapy for myeloid leukemias: a phase I trial of bismuth-213-HuM195 (anti-CD33) [abstract]." Blood. 1997;90:2245.
10. Parker C, Nilsson S, Heinrich, et al. "Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer." N Engl J Med. 2013;369:213-223.
11. Nguyen C, Faraggi M, Giraudet AL, et al. "Long-term efficacy of radionuclide therapy with disseminated neuroendocrine tumors uncontrolled by conventional therapy." J Nucl Med. 2004;45:1660-1668.
12. Forrer F, Uusijarvi H, Storch D, Maecke HR, Mueller-Brand J. "Treatment with 177Lu-DOTATOC of patients with relapse of neuroendocrine tumors after treatment with 90Y-DOTATOC." J Nucl Med. 2005;46:1310-1316.
Katherine Zukotynski is a staff radiologist at Sunnybrook Health Sciences Centre in Toronto and an assistant professor in the faculty of medicine at the University of Toronto. She serves on the Society of Nuclear Medicine and Molecular Imaging (SNMMI) Radioisotope Therapy Outreach Working Group and has been involved with the PET Center of Excellence since 2011. She completed the SNMMI Future Leaders Academy in January 2015. Her main area of research has been in PET/CT and oncology.


 April 18, 2024
April 18, 2024