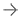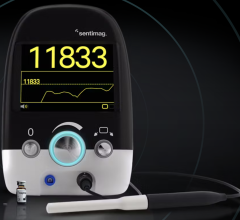
December 3, 2018 — GE Healthcare recently launched the Invenia automated breast ultrasound (ABUS) 2.0 system in the United States. This device is the only U.S. Food and Drug Administration (FDA)-approved ultrasound supplemental breast screening technology, according to GE, specifically designed for detecting cancer in dense breast tissue. When used in addition to mammography, Invenia ABUS can improve breast cancer detection by 55 percent over mammography[1] alone.[2]
“We are committed to informing patients about breast density, and offering supplemental screening options,” said Sophia Roumanis, M.D., section head of breast imaging and intervention, Beaumont Breast Care Center, Dearborn, Mich. “We are thrilled to add this advanced ultrasound technology to our breast cancer screening program, which allows better visibility of dense breast tissue during breast cancer screenings.”
More than 40 percent of women in the U.S. have dense breast tissue[3], which is one of the strongest common risk factors for developing breast cancer. Breast density is a measurement of the amount of fatty tissue versus the amount of fibrous tissue in the breast. Both cancer and dense tissue appear white on a mammogram, so looking for tumors in women with dense breasts can be like looking for a snowball in a snowstorm. Because of this, tumors are often unseen – or “masked” – on mammography exams.
“Our goal is to find cancers as early as possible to offer the best potential outcome for the patient,” commented Vidya Pai, M.D., section head of breast imaging and intervention, Beaumont Hospital, Royal Oak, Mich. “By offering this supplemental screening to mammography for patients with dense breast tissue, we anticipate improving detection for small cancers that may not be seen on a mammogram alone in these women.”
Launched in 2014, Invenia ABUS has been installed at hundreds of facilities around the world. The new Invenia ABUS 2.0 builds on its predecessor to enhance the exam experience for both operators and patients, including new features that further customize the exam based on the patient’s body:
-
Efficient exams: The cSound Imageformer, a software-based graphics processor, provides a reproducible and operator-independent acquisition method to achieve consistent, high-quality results. cSound imaging allows significantly more data to be collected and used to create every image. Traditional hand-held ultrasound parameters such as focal zones and gain are automatically optimized. No image manipulation is required, so that high image quality is consistent from operator to operator with the touch of a button; and
-
Improving the patient experience: The gentle shape of the Reverse Curve transducer follows the natural contour of the breast, providing patient comfort, thorough contact and helping ensure comprehensive coverage. The 15 cm large field-of-view transducer is easy to position and maintains even compression while scanning. Since no two women are identical, exams can be customized with programmable scan protocols, adjustable scan depths and compression levels. With the touch of a button, the operator can also shorten scan time once breast tissue acquisition is complete.
For more information: www.gehealthcare.com
References
[1] Increase in cancer detection associated with an overall decrease in false positives.
[2] Brem RF, Tabár L, et.al. Assessing Improvement in Detection of Breast Cancer with Three-dimensional Automated Breast US in Women with Dense Breast Tissue: The SomoInsight Study. Radiology. 2015 Mar; 274(3): 663-73.
[3] https://ww5.komen.org/Breastcancer/Highbreastdensityonmammogram.html

 May 13, 2024
May 13, 2024