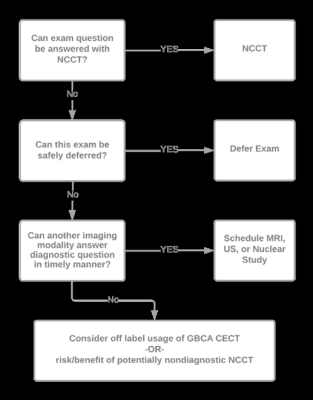
Decision tree for manual review of currently scheduled contrast-enhanced CT examinations in the event that iodinated contrast supply is critically low. CECT = contrast-enhanced CT. GBCA = gadolinium-based contrast agent. NCCT = non-contrast CT. US = ultrasound.
May 18, 2022 —According to ARRS’ American Journal of Roentgenology (AJR), to ensure that patients with emergent or life-threatening imaging indications can continue receiving care, radiology practice leaders need to quickly assess their contrast inventories, prioritize examination findings, and reduce overall iodinated contrast media (ICM) usage.
“Establishing awareness of the ICM shortage throughout the hospital system is an important first step,” wrote coauthors Joseph Cavallo, MD, MBA, and Jay Pahade, MD, from the department of radiology and biomedical imaging at Yale’s School of Medicine.
Alerting frequent referrers for CECT (ED, oncology, surgery) and concomitant users of ICM (cardiology, vascular surgery, GI, radiation oncology, urology) can align preservation strategies throughout the institution.
Additionally, common indications for CECT can be stratified, according to necessity, with three primary strategies for reducing ICM usage:
- direct ICM dose reduction;
- alternative diagnostic imaging modalities or CT contrast agents;
- deferment of imaging.
Drs. Cavallo and Pahade also provide examples of dose reduction that have been implemented at their institution—noting that the feasibility of any dose reduction depends on the specific equipment and imaging protocols at individual facilities.
“Unfortunately,” the two AJR authors added, “departments may need to resort to deferment of CECT examinations.” Ideally, rescheduling should be limited to certain low-risk examinations (i.e., annual staging examinations in patients without clinical evidence of recurrence), as well as follow-up examinations of incidental findings not posing an immediate threat to the patient.
However, in the most critical of shortages, manual review of all pending CECT examinations may be necessary.
The unanticipated, albeit severe shortage in ICM currently affecting practices around the world is expected to continue into late June of this year.
For more information: www.arrs.org
Related Content of MRI Gadolinium Safety Concerns
Voluntary Dismissal of Chuck Norris Gadolinium Case Involving Bracco
VIDEO: How Serious is MRI Gadolinium Retention in the Brain and Body? An interview with Max Wintermark, M.D.
VIDEO “Big Concerns Remain for MRI Gadolinium Contrast Safety at RSNA 2017,” An interview with Emanuel Kanal, M.D.
Radiology Has Failed to Properly Assess or Track MRI Gadolinium Contrast Safety
Recent Developments in Contrast Media
FDA Committee Votes to Expand Warning Labels on Gadolinium-Based Contrast Agents
European Medicines Agency Issues Update on Gadolinium Contrast Agents


 May 17, 2024
May 17, 2024