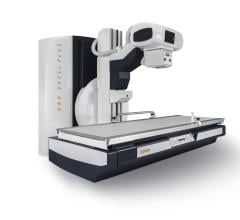
November 4, 2014 — Toshiba America Medical Systems Inc.’s Kalare radiographic and fluoroscopy (R/F) digital X-ray system with flat panel detector (FPD) has received U.S. Food and Drug Administration (FDA) clearance.
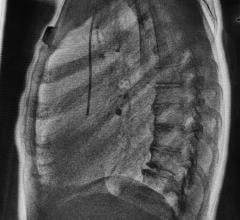
The enclosed table design of the Kalare FPD R&F system may reduce scatter radiation by up to 95 percent, optimizing safety for patients and staff, while its 17-inch x 17-inch size provides quality images edge-to-edge without an increase in dosage. This design also helps reduce panning time for exams requiring fluoroscopy, such as a barium swallow and eliminates periphery image distortion, leading to higher image quality and better diagnoses.
The Kalare R&F digital X-ray system will be showcased at this year’s Radiological Society of North America (RSNA) annual meeting in Chicago, Nov. 30–Dec. 5, 2014.
For more information: www.medical.toshiba.com


 April 01, 2024
April 01, 2024