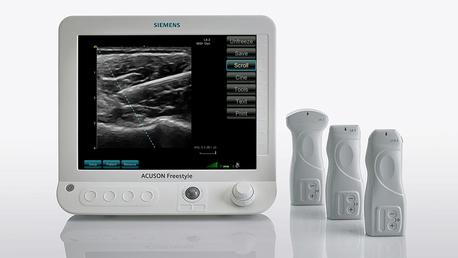
October 13, 2014 — Siemens Healthcare has introduced Release 3.5 of the Acuson Freestyle ultrasound system — the world’s first ultrasound system with wireless transducers — to enhance clinical and operational efficiencies at the point of care. With excellent image quality at the pixel level courtesy of Pixelformer image processing architecture, this updated version of the Acuson Freestyle system covers a wide range of ultrasound imaging for diagnostic and procedural cases, including vascular, musculoskeletal, and nerve imaging. New enhanced needle visualization improves needle display during ultrasound-guided procedures. A new user interface offers improved ease-of-use with a larger clinical image display, auto-hide of imaging parameters, preset display customization, and an up-to-60-second clip playback feature.
Release 3.5 of the Acuson Freestyle system also introduces a mobile link app that connects the ultrasound system to a Microsoft Windows tablet [1] on a shared wireless network to view, open, and share images and clips within the stored Patient Study List. Via the app, system users can create patient study work lists and send them to the Acuson Freestyle system to begin new studies more quickly and easily.
For more information: www.siemens.com/freestyle
References:
1. Operates on a Microsoft tablet device running Windows operating system 8.1 or higher. For informational purposes only. Not intended for diagnostic use. The products/features mentioned in this document may not be commercially available in all countries. Due to regulatory reasons their future availability cannot be guaranteed.


 November 03, 2025
November 03, 2025 









