March 16, 2010 - Physicians took new steps toward understanding what patient type would benefit most from Yttrium-90 radioembolization treatment for hepatocellular carcinoma (HCC).
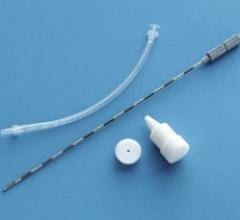
Radioembolization treatment, commercially known as TheraSphere, is a liver cancer therapy that consists of millions of small glass beads (20 to 30 micrometers in diameter)containing radioactive yttrium-90. The product is injected by physicians into the main artery of the patient's liver through a catheter, which allows the treatment to be delivered directly to the tumor via blood vessels.
Doctors presented their results the 2010 Society of Interventional Radiology (SIR) 35th Annual Scientific Meeting. In one of the presentations, Riad Salem, M.D., interventional radiologist and professor of radiology, medicine and surgery at Northwestern University, Chicago, Ill., and Robert Lewandowski, M.D., a co-author and an interventional radiologist at Northwestern Memorial Hospital, explained how they assessed clinical outcomes utilizing TheraSphere in 291 patients suffering from HCC. presented a comprehensive analysis of the findings.
Dr. Salem said they were able to determine what particular patient type would benefit from this treatment, and that the study could support future studies of similar scope.
Steve Hong, M.D., a radiologist at William Beaumont Hospital, Royal Oak, Michigan, presented a study addressing the deterioration in liver function following radioembolization treatment for HCC, for which the principal investigator was Michael Savin, M.D., an interventional radiology researcher also from William Beaumont Hospital. The study concluded that while liver deterioration is a known complication of radioembolization, most liver deterioration appeared to occur due to underlying cirrhosis or further progression of the patient's liver cancer.
For more information: www.mdsnordion.com/Therasphere

 April 29, 2024
April 29, 2024