December 22, 2023 — Quibim, a company pioneering imaging biomarkers for Precision Medicine that has created AI-based software to identify early-stage neurodegenerative diseases, is pleased to announce today the launch of QP-Brain. This follows the product receiving an FDA 510(k) clearance from the US Food and Drug Administration, a CE mark for the European Union and a UKCA mark for the United Kingdom. This means the product is now cleared to be used by clinicians in those markets.
With up to one billion individuals affected by neurological diseases worldwide, plus global increases in the number of people reaching an older age when neuro-diseases are more common, the demand for quantitative assessments to detect early-stage changes in the brain has never been higher.
Currently, the vast majority of disease detection in this field comes from qualitative analysis from radiologists and neurologists. This solely human-based approach means that neuro diseases are often only detected in their later stages. In order to resolve the issue, healthcare institutions require advanced and versatile quantitative AI-based tools that can also provide secure transfer and storage of patients’ data and augment the workflow of clinicians.
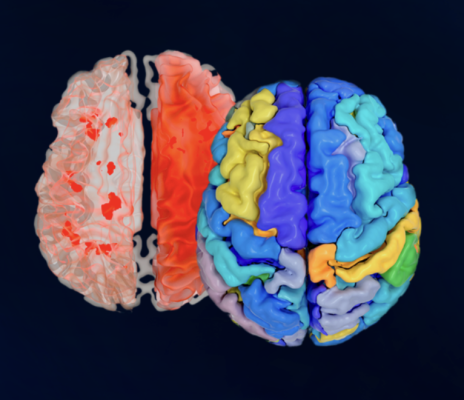
QP-Brainwas therefore designed by Quibim to facilitate quantitative analysis of patients’ brains by using an AI-based tool that automatically quantifies and displays results based on the data from MRI Scans directly in PACS. The technology measures brain regions to detect subtle alterations and provides quantitative information to healthcare professionals which, in turn, optimizes the process of radiological reporting.
Additionally, Quibim’s new product provides quantification and visualization of imaging findings. This is useful for identifying patterns of brain atrophy and neurodegenerative diseases like Alzheimer’s, multiple sclerosis, and vascular and frontotemporal dementia, at earlier stages and also makes reporting more effective.
Designed with data safety in mind, QP-Brain is compliant with both the EU General Data Protection Regulation (GDPR) and the US Health Insurance Portability and Accountability Act (HIPAA). It offers hospitals a secure way of processing and analyzing patient data and can be easily installed on a hospital server behind firewalls through a secure cloud architecture.
Dr. Angel Alberich Bayarri, Quibim’s founder and CEO, commented: “Receiving regulatory clearance for QP-Brain® in the US, EU and UK is a huge milestone for Quibim and for the early-stage detection of neurological diseases. At Quibim our mission is to develop AI technology that augments the essential role played by human medical experts to improve patient outcomes, by detecting neurological diseases and cancers much earlier than is currently possible.”

 April 25, 2024
April 25, 2024 








