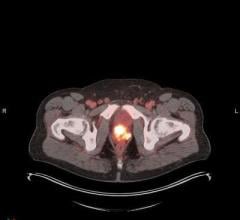
October 23, 2015—Patients with intermediate meningiomas treated with radiation therapy (RT) after surgery experienced a 96 percent three-year progression-free survival rate and had minimal adverse events, according to new research. Study findings were presented at the American Society for Radiation Oncology’s (ASTRO’s) 57th annual meeting.
Meningioma, the most common type of central nervous system tumor, nearly always forms on the meninges—the membranes that surround the brain and spinal cord. Most meningiomas, approximately 75 percent, are benign (noncancerous) and may not require immediate treatment. However, many need to be removed because of proximity to or impingement of critical structures, tumor growth or because they are not benign (in other words, they are atypical or anaplastic). Treatment decisions for patients with meningioma depend on many factors, with the more advanced meningiomas often requiring a combination of surgery and RT.
RTOG 0539, the first successfully completed cooperative group meningioma trial, enrolled 244 patients from sites throughout the United States and Canada. The study’s aim was to estimate patients’ three-year progression-free survival (PFS) rates in each of the study’s assigned patient groups. PFS indicates the length of time during and after the treatment of a disease that a patient lives with the disease but it does not get worse. The pathology and imaging reports of patients in the trial were all centrally reviewed and patients were assigned to three different groups depending on prognosis and management strategies, which were based upon tumor grade, recurrence status of the tumors and the extent of the surgical removal.
In this first analysis of RTOG 0539, researchers looked at the three-year PFS and adverse events for the intermediate-risk group of patients (defined as Group 2). Patients in Group 2 had either a newly diagnosed grade II meningioma (atypical, meaning neither frankly malignant nor benign) with gross total resection (GTR, Simpson I-III), or a recurrent grade I (benign) meningioma, meaning that it had been previously treated with surgery but had subsequently progressed. Any degree of surgical resection was permitted for patients with a recurrent grade I tumor. The phase II study compared the patients to a predefined historical control group of intermediate-risk meningiomas.
Of the 56 original patients classified to Group 2, 52 patients received protocol treatment (three were ineligible and one did not receive RT). Of these 52 patients, 36 (69.2 percent) were classified as having WHO grade II tumors with GTR; while 16 patients (30.8 percent) had recurrent grade I tumors. The patients received RT of 54 Gy in 30 fractions post-surgically. While 3-D conformal radiation therapy (CRT) was permitted within the study guidelines, the majority of patients (84.6 percent) received intensity-modulated radiation therapy (IMRT). This was the first RTOG brain trial with protocol-specific IMRT parameters. Four of the 52 patients withdrew (without recurrence), so 48 patients were evaluable for the primary endpoint of three-year PFS.
Data indicated that there was no difference in PFS between the subgroups (P=.503), validating the study’s co-grouping of the patients into one prognostic category. The three-year PFS for Group 2 was 96 percent. One patient with a WHO grade II tumor died from the disease and one patient with a WHO grade I tumor died from an undetermined cause without disease progression.
Adverse events were scored using NCI common toxicity criteria, and the study specifically measured grade one or grade two adverse events. Among the 44 patients who received IMRT, four patients (9 percent) developed grade 2 acute adverse events, and 11 patients (25 percent) had grade 2 late adverse events.
“Our results have been the impetus for phase III trials and support the use of postoperative radiation for intermediate-risk meningiomas, in addition to documenting positive outcomes of using IMRT,” said lead study author Leland Rogers, M.D., professor in the radiation oncology department at Virginia Commonwealth University. “We are gaining knowledge about meningiomas at a more rapid pace. This study has shown we can successfully treat meningioma patients in a large, cooperative group setting and can achieve excellent outcomes with surgery and RT.”
For more information: www.astro.org


 May 16, 2024
May 16, 2024