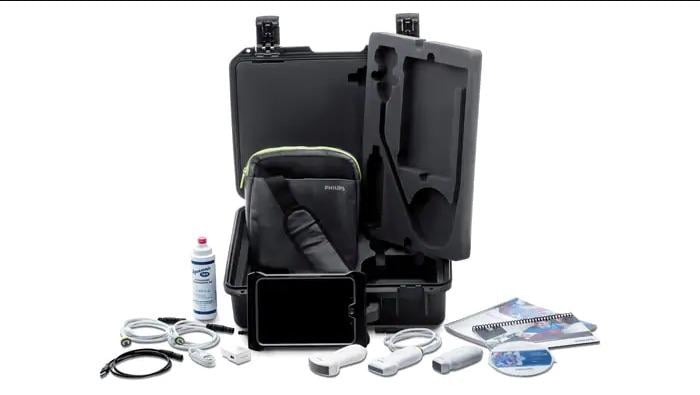
May 22, 2019 — Philips announced that Philips Lumify, a point-of-care ultrasound device, has earned the U.S. Army Airworthiness Certification after passing a series of rigorous military tests. This certification now allows Philips Lumify to be added to an Army medic’s bag in a helicopter so they can immediately evaluate if a solider has a life-threatening injury, such as internal bleeding, a broken rib or a bullet wound to critical organs. The Airworthiness Certification also allows medics using Philips Lumify to facilitate continuity of care between the battlefield, aircraft, ambulance and emergency room.
To receive Airworthiness Certification, medical carry-on devices must pass rigorous tests to meet military standards. These tests and evaluations include human factors, securing procedures, battery performance, electrical safety, low pressure and rapid decompression, environmental climate tests, explosions and more.
For more information: www.usa.philips.com/healthcare

 April 24, 2024
April 24, 2024