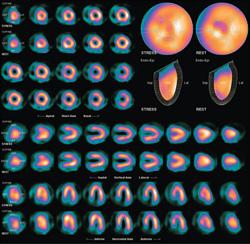
June 12, 2013 — UltraSPECT, a provider of nuclear medicine (NM) image reconstruction technology that reduces radiopharmaceutical dose and acquisition time, announced the installation of its proprietary Wide Beam Reconstruction (WBR) software at nearly 10 healthcare facilities. The installations came as a result of the recent agreement between UltraSPECT and radiopharmaceutical provider PharmaLogic, under which PharmaLogic offers the WBR software as part of its patient-centered approach.
WBR addresses concerns about patient radiation exposure and improved image quality. It enhances the performance of existing NM cameras, enabling a 50 percent reduction in ASNC-recommended radiopharmaceutical injected dose, reducing imaging scan time up to 50 percent while maintaining the full level of image quality. The technology, sold as part of the UltraSPECT Xpress.Cardiac, Xpress3.Cardiac and Xpress.Bone products, can be utilized with most major manufacturers’ NM systems, as well as all clinical software packages.
"The industry’s growing interest to reduce radiation exposure to patients and to the technologists performing the exams led us to look for a cost-effective, low-dose solution, resulting in our incredibly successful partnership with UltraSPECT,” said Steve Chilinski, president and CEO of PharmaLogic. “We believe these initial installations are just the start of a growing demand for the solution as all of our nuclear medicine customers see that everyone from patients and technologists to physicians, pharmacies and pharmacy staff will benefit.”
UltraSPECT is the only company to offer the proprietary software solution — approved by the U.S. Food and Drug Administration (FDA) — that can be retrofit into existing SPECT (single-photon emission computed tomography) imaging systems. The UltraSPECT Xpress line of products can be readily installed on current gamma camera systems. Utilizing the proprietary WBR iterative reconstruction algorithm, which simultaneously addresses resolution recovery and noise reduction for low-count density, it provides high image resolution for greater lesion localization, raising diagnostic confidence and precision. The products minimize radiation exposure for both patients and technologists, while abbreviated acquisition times reduce the possibility of patient motion artifacts and enhance patient throughput and comfort. For many WBR users, the result is enhanced equipment utilization, revenues, patient satisfaction and referrals.
Huntington Internal Medicine Group (HIMG), Huntington, W.Va., recently selected the UltraSPECT Xpress.Cardiac and Xpress.Bone solutions as part of their partnership with PharmaLogic. "When we learned about UltraSPECT’s WBR technology, we knew immediately that we needed it, and the results have been even better than what we had expected,” said Juanita Dempsey, cardiology and radiology manager at HIMG. “Not only does the ability of the software to enable half of the radioactive dose help protect our patients, but it’s greatly protecting our technologists who are overseeing the procedures again and again."
UltraSPECT is highlighting its WBR software at the annual meeting of the Society of Nuclear Medicine and Molecular Imaging (SNMMI), being held in June 9-12 in Vancouver, British Columbia.
For more information: www.ultraspect.com


 April 10, 2024
April 10, 2024 





![(A) PET images of [68Ga]Ga-DOTA-ZCAM241 uptake at baseline and 3, 7, and 12 days after injection as inflammatory arthritis developed in single representative individual mouse. Images are normalized to SUV of 0.5 for direct comparison between time points. (B) CD69 immunofluorescence Sytox (Thermo Fisher Scientific) staining of joints of representative animals during matching time points.](/sites/default/files/styles/feed_medium/public/PET%20Tracers.jpeg?itok=P5Di6MIe)

