March 29, 2011 – A two-step platform has been released for use with C-arm digital radiography (DR) systems. The platform is made by Clear Image Devices.
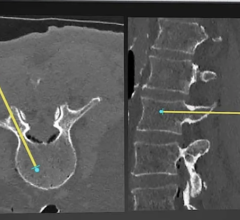
The device enables imaging technicians to place the patient in the optimal position for safe, easy capture of the highest quality weight-bearing AP foot images with C-Arm systems. Dual handrails and locking casters ensure a stable, safe platform and permit patients to be positioned over a variety of C-arm DR systems including those made by Swissray, IMIX, and others.
The base of the platform includes an X-ray lucent faceplate made of unbreakable polycarbonate designed to support patients weighing up to 500 pounds. The platform is designed for the most practical use in any lab environment: easily portable and easy to keep clean, and made with germ-resistant, non-porous polyethylene and steel.
For more information: www.clearimagedevices.com


 March 19, 2025
March 19, 2025