July 5, 2019 — Medic Vision Imaging Solutions announced that its 3-D iterative image reconstruction technology for shortening magnetic resonance imaging (MRI) scans, iQMR, has been cleared by the Japanese Pharmaceuticals and Medical Devices Agency (PMDA). The software will be distributed in Japan by Nagase & Co. Ltd.
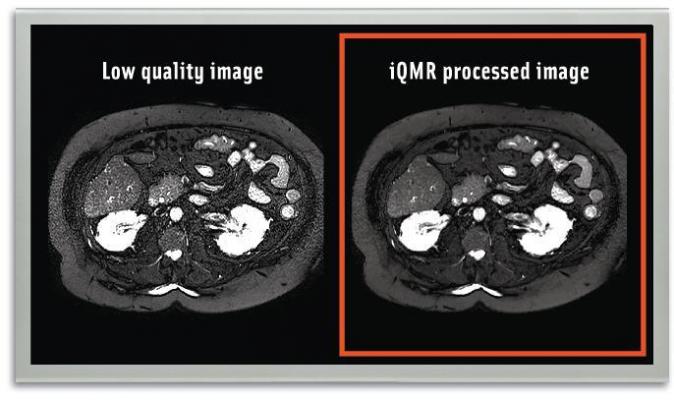
The iQMR system enables short MRI protocols and enhanced image quality, to facilitate increased productivity, fewer repeating scans and improved patients’ experience.
According to the Organisation for Economic Cooperation and Development (OECD) there are approximately 7,000 MRI scanners in Japan, second only to the U.S. market (with approximately 12,000 scanners). The number of MRI scanners per 1 million population in Japan is the highest among 27 OECD countries (51.7 versus a mean of 21.1). The number of MRI annual exams per 1,000 population in Japan is secnd among 17 OECD countries (112 versus a mean of 64).
“The growing demand of high-quality MRI scans in the Japanese healthcare system, encouraged us to introduce local hospitals and medical imaging centers [to] a solution that allows enhanced productivity, better diagnostic outcome and experience for their patients,” said Nahomu Kameda, manager of the Medical Business Acceleration Team of Nagase & Co. Ltd.
“We have been collaborating with Medic Vision since 2016. Its low-dose solution for CT [computed tomography] imaging, SafeCT, was cleared by PMDA in 2017, and is being practiced by hospitals and facilities in Japan since then. We anticipate that iQMR will revolutionize the Japanese MRI market with its image enhancement and fast MRI capabilities. These abilities that can be performed on any scanner, are essential to the local medical imaging centers,” continued Kameda.
Medic Vision CEO Eyal Aharon said that iQMR has allowed facilities in the U.S. and China to reduce their MRI scan time by 38 percent on average in the past year and acquire neuro MRI scans in five minutes overall.
For more information: www.medicvision.com


 April 24, 2024
April 24, 2024