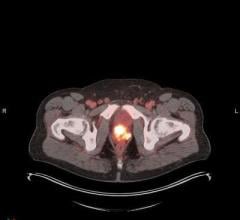
18F-NaF PET image.
March 19, 2010 - To alleviate the effects of the global molybdenum shortage, a national network of 47 radiopharmacies will receive 18F Sodium Fluoride (NaF) free of charge from between March 22 and April 9, 2010.
Participants in this trial program will receive 18F NaF, provided by PETNET Solutions Inc., a subsidiary of Siemens Medical Solutions USA, for only a nominal delivery fee.
18F NaF is an FDA-approved imaging agent and is used in the detection of bone metastases. As 18F NaF is not currently covered by Medicare or Medicaid, ubmission of NaF scan claims to other insurers is permitted only if the plan clearly covers NaF PET bone scans. As a condition of participation, the provider cannot charge the patient for the NaF dose.
The supplier anticipates 18F NaF use to grow due to its ability to deliver noticeably better image quality than traditional planar bone scans, and to offer greater differentiation of benign versus malignant lesions. PETNET Solutions is prepared to distribute 18F NaF as this demand increases.
CMS reimbursement support of the imaging agent could provide the medical community with alternatives to 99mTc labeled diphosphonate agents, in particular, during the ongoing global shortage of 99Mo/99mTc generators.
For more information: www.siemens.com/healthcare


 May 17, 2024
May 17, 2024