March 23, 2016 — Florida Cancer Affiliates (FCA) is helping to pioneer a new tool aimed to protect prostate cancer patients from the negative effects of radiation therapy. Recently a patient, one of the first in Florida, was injected at FCA with SpaceOAR hydrogel, the first U.S. Food and Drug Administration (FDA)-cleared spacing device to protect the rectum in men undergoing radiation therapy for prostate cancer.
The SpaceOAR System is intended to temporarily position the anterior rectal wall away from the prostate during radiotherapy for prostate cancer, creating space to protect the rectum from radiation exposure. Lawrence Hochman, M.D., and Sanjay Emandi, M.D., of FCA are the first radiation oncologists in the state certified to safely administer the SpaceOar hydrogel.
“Providing the best possible treatment to patients is our top priority, which is why we are one of the first centers offering SpaceOAR hydrogel,” said Hochman, DO, FACRO, practice president, FCA-Tampa Bay. “FCA has a long history of bringing new technological advances for radiation therapy to the local communities in Florida. We pride ourselves with providing patients with the cutting-edge and high-quality treatment options they deserve.”
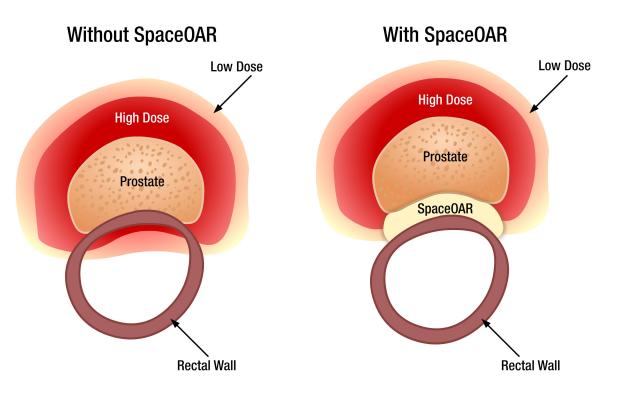
Because of the close proximity of the prostate to the rectum, prostate radiation therapy typically results in some radiation hitting the rectum, which can sometimes cause side effects. The SpaceOAR System creates space and pushes the rectum away from the prostate and the high dose area. Placed through a small needle, the hydrogel is administered as a liquid, but quickly solidifies into a soft gel that expands the space between the prostate and rectum. The hydrogel spacer maintains this space until radiation therapy is complete. The spacer then liquefies and is absorbed and cleared from the body in the patient’s urine.
“Creating space between the prostate and rectum is an important technological advance that significantly protects the rectum during radiation treatments and reduces the likelihood of early and late rectal side effects,” emphasized Emandi, radiation oncologist, FCA. “Men facing prostate cancer may have some difficult decisions to make, but utilizing SpaceOAR hydrogel during radiation therapy should not be one of them. We have successfully implemented the option of using the Space OAR hydrogel into the treatment of our prostate cancer patients and have seen excellent results.”
According to the American Cancer Society and the National Cancer Institute, prostate cancer is second only to skin cancer as the most frequently diagnosed cancer in men, with an estimated 220,800 new cases and 27,540 deaths in the United States in 2015 alone. Worldwide, prostate cancer is expected to grow to 1.7 million new cases and 499,000 deaths by 2030.
FDA clearance was granted following completion of the SpaceOAR System prospective, multicenter, randomized clinical trial. SpaceOAR patients experienced a significant reduction in rectal radiation dose and severity of late rectal toxicity when compared to control patients who did not receive SpaceOAR hydrogel. The results of the SpaceOAR pivotal clinical trial were published in the August 2015 edition of the International Journal of Radiation Oncology.
For more information: www.spaceoar.com


 April 10, 2024
April 10, 2024