April 15, 2014 — The U.S. Food and Drug Administration (FDA) granted 510(k) market clearance for the GE Healthcare Revolution computed tomography (CT) system. The 256-slice CT system offers technology that provides excellent image quality and clinical capabilities through the convergence of coverage, spatial resolution and temporal resolution.
CT still has challenges with patients that have high heart rate, metallic implants, and non-compliant patients. The Revolution CT is able to address these challenging patients by freezing cardiac motion in one heartbeat, reducing metal artifacts, and offering the potential for sedation-free CT scanning. The system also offers benefits for sensitive patient groups such as pediatric, renal insufficiency, trauma, and stroke.
"I had the opportunity to gather the clinical images for the submission of Revolution CT to the FDA and I have been impressed ever since," said Ricardo C. Cury, M.D., chairman of radiology and director of cardiac imaging at Baptist Health South Florida, who served as principal investigator for gathering clinical images. "Diagnostic quality images are now possible in challenging patients like those with high heart rates, which is a significant advancement."
Revolution CT's convergence of technological advances include 16 cm whole organ coverage, best-in-class spatial resolution through the new Gemstone Clarity Detector, and a new gantry designed to image at 0.28 second rotation speed and tested to support rotation speeds up to 0.2 seconds in the future.
The impact of the technology includes:
• Cardiology: One beat, motion-free cardiac in high definition at any heart rate with or without beta-blockers. This delivers the clinical information needed for the coronaries, myocardial perfusion and function with one contrast injection.
• Oncology: Low-dose, whole organ diagnosis and follow up of organs such as the liver, kidneys, pancreas, etc. are enabled by dynamic acquisition modes.
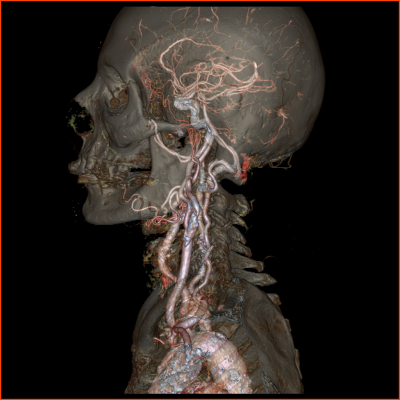
• Neurology: Rapid, comprehensive stroke assessment with whole brain perfusion and dynamic CT angiography at very low dose.
"This system should bring us to another level of image quality," said Lawrence Tanenbaum, M.D., FACR, associate professor of radiology and the director of MRI, CT and outpatient/advanced imaging development at Mount Sinai School of Medicine in New York. "With Revolution CT, I expect to see better routine images and more simple access to the more advanced procedures."
ASiR-V, GE's next generation of iterative reconstruction technology also recently cleared by FDA, is designed to deliver reduced noise levels, improve low-contrast detectability, and routinely reduce dose up to 82 percent for patients of all ages with uncompromised image quality. This especially benefits pediatric and other patients considered more sensitive to radiation exposure. Clinicians can reduce patient anxiety with Revolution CT's Whisper Drive, soft ambient lighting, personalized gantry display, and bore pattern. Fast and low dose 70 kVp acquisitions for sedation free and minimal breath hold pediatric studies are also now possible.
"This will be the first CT scanner that's right for physicians in every clinical specialty and provides answers from one CT exam" said Steve Gray, president and CEO of GE Healthcare MICT. "Revolution CT is able to scan even the most challenging patients, day in and day out, with remarkably clear images. It can also scan pediatric patients at very low doses. And, we made sure that using it is productive, logical, and intuitive."
GE Healthcare plans worldwide commercial shipments of the Revolution CT this summer.