March 1, 2023 — MRIguidance received FDA clearance for its BoneMRI cloud solution as well as for its extended support for the use of BoneMRI Lumbar Spine on GE 1.5T scanners. This clearance makes BoneMRI accessible to the majority of relevant diagnostic MRI scanners in the US; and the cloud solution brings several benefits into the clinical workflow.
The clearance expands the availability of BoneMRI in the US market where Lumbar Spine was already cleared last November 2022 for Siemens 1.5 & 3.0 T. Additionally, BoneMRI Pelvic Region was FDA cleared in December 2021 and is available for Philips & Siemens 3.0 T in the US.
BoneMRI Cloud
The FDA clearance includes BoneMRI Cloud, bringing several benefits to healthcare providers including:
✓ Faster installation and upgrading processes
✓ Reduced maintenance and support effort for local IT staff
✓ No need for dedicated and specific hardware investments
✓ Faster BoneMRI reconstructions
Additionally, BoneMRI Cloud allows healthcare providers a more flexible and convenient workflow that (1) begins with the introduction of the BoneMRI protocol, (2) followed by the scanning of the patient and the acquisition of MR data. (3) The MR data is then sent to the PACS, where it (4) crosses a gateway and is pseudonymized. From there, (5) the data reaches the cloud, and a BoneMRI reconstruction is created. (6) The resulting BoneMRI image is sent back to PACS and combined with the MR data before being sent to the (7) radiology workstation.
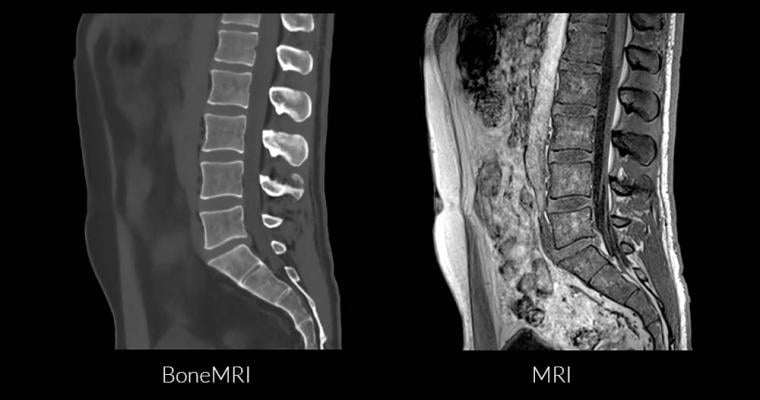
This streamlined workflow provides radiologists with aligned soft tissue and bone images, which are ideal for diagnostics.
This clearance further reinforces MRIguidance commitment to fully exploit the technological possibilities in medical imaging to benefit value-based healthcare and deliver safe patient care.
BoneMRI in Europe
BoneMRI is also available in the European market and is CE marked for Lumbar Spine, Cervical Spine and Pelvic Region with the cloud feature included.
About MRIguidance
MRIguidance is a medical imaging software company that develops and markets BoneMRI, the world’s first imaging technique that visualizes both bone and soft-tissue from one imaging exam without the use of harmful radiation.
The team at MRIguidance works in close collaboration with hospitals to develop and clinically validate BoneMRI.
For more information: https://mriguidance.com/


 May 13, 2024
May 13, 2024