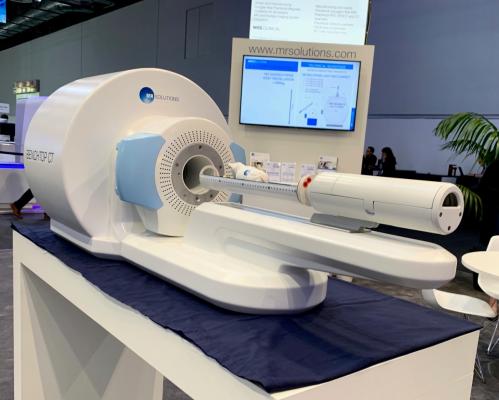
February 28, 2020 — MR Solutions will be displaying its latest dry magnet 3T to 9.4T preclinical, multi-modality MRI (with PET or SPECT) and new benchtop PET/CT systems to the imaging science community at the 15th European Molecular Imaging Meeting (EMIM 2020) on March 24-27, 2020 in Thessaloniki, Greece.
Fabrice Chaumard, MR Solution’s sales and marketing director commented: “Our very latest compact scanners with multi-modality imaging techniques will be on display at EMIM. These systems offer better performance at a very competitive cost and do not require helium top-ups. We will be welcoming visitors to Booth #104 to show off our state of the art range of MRI/PET/SPECT/CT technology with interchangeable cradles to speed-up and minimise handling.”
MR Solutions’ preclinical MRI systems offer superior soft tissue contrast and molecular imaging capability for great visualisation, quantification and translational studies. Because the molecular imaging systems are compact they do not need a reinforced concrete floor, Faraday cage, liquid helium, or special height requirement for the room. They can also be installed next to other scanners and other sensitive equipment as their stray magnetic field is a few centimetres compared to metres with liquid helium cooled systems.
MR Solutions’ MRI and CT technology can be combined with PET or SPECT imaging modules for either simultaneous or sequential imaging (dependent on the chosen configuration).
The EMIM is an annual event organized by the European Society for Molecular Imaging. The programme allows researchers to discuss and share knowledge within the molecular imaging community and to take a look at emerging technologies.
MR Solutions works closely with leading academic institutions across the world to be at the cutting edge of performance. Its own research, development and assembly of the scanners is carried out in the UK with 99% of their orders being exported to the world’s leading academic and research institutions.
MR Solutions’ dry magnet, preclinical MRI systems have been recognized with prestigious award wins including three Queen’s Awards for Enterprise – for innovation in 2016 and 2019 and for international trade in 2017.
For more information: www.mrsolutions.com


 April 24, 2024
April 24, 2024