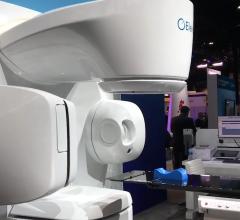
February 26, 2009 - Accuray Inc. said its recent 8th Annual CyberKnife Users' Meeting attracted over 500 surgeons, radiation oncologists, medical physicists, hospital administrators and other related clinical staff, many of whom gave presentations on the use of the CyberKnife Robotic Radiosurgery System for the noninvasive treatment of tumors anywhere in the body.
At this year's meeting, nine presentations reported on large-scale studies, each with more than 100 patients with brain, lung or prostate tumors. The 2009 Users' Meeting included 11 presentations related to prostate cancer treatment, with follow-up data approaching three years for many patients. In addition to updated outcomes with longer follow-up, researchers are extending CyberKnife applications for prostate cancer beyond early-stage disease. For example, clinicians discussed their use of CyberKnife radiosurgery to "boost" the radiation dose after conventional radiation therapy for patients with advanced disease, as well as for the treatment of recurrent cancer in patients that failed conventional radiation therapy.
The adoption of CyberKnife radiosurgery for the treatment of lung cancer continues to grow, a trend that was supported by 11 clinical lung cancer presentations. Presenters reported on primary and metastatic lung cancer, the treatment of recurrence after radiation therapy and chemotherapy, and quality of life after lung cancer treatment. In one study, patients showed 94 percent local control of lung tumors at a median follow-up of 33 months.
CyberKnife advantages continue to be extended in brain cancer and other brain disorders, according to the company. Multiple presentations highlighted the CyberKnife System's frameless capability to conveniently treat in multiple sessions — a particularly beneficial capability when lesions are near sensitive critical structures such as those that control vision and hearing. In one study of 114 acoustic neuroma patients, the authors concluded that the CyberKnife System's abilities made radiosurgical treatment possible for more than 13 percent of patients who could not have been treated with single-session radiosurgery because of risk of hearing loss.
For more information: www.accuray.com

 April 29, 2024
April 29, 2024