Calypso Medical Technologies Inc. announced that its FDA-cleared Calypso 4D Localization System and Beacon electromagnetic transponder manufacturing process, used to monitor organ motion in cancer patients, has been certified by the International Organization for Standardization (ISO).
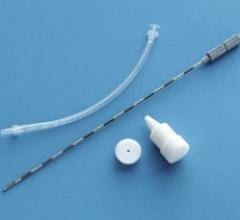
The system utilizes proprietary electromagnetic technology in conjunction with implanted Beacon transponders in the prostate. The product was designed to provide tumor location information during external beam radiation therapy without adding ionizing radiation.
During radiation therapy, the system can monitor organ motion for cancer patients. With clinicians expected to have greater knowledge of tumor location, it may ensure that the radiation beam is always on target. The product does not require expertise in interpreting X-ray or ultrasound images, so it reduces the time to perform patient setup.
ISO sets standards ensuring a comprehensive management system is used for the design and manufacture of medical devices. The certification status by the ISO demonstrates Calypso Medical meets an international set of standards and guidelines that are becoming vital components of business transactions, global trade and regulatory requirements.


 May 14, 2024
May 14, 2024