August 9, 2022 — Bot Image, an Omaha-based MRI medical device company has developed an AI-driven medical device CAD software to significantly improve the accuracy and speed of prostate cancer detection (CADe) and diagnosis (CADx). The tool, called ProstatID, combines artificial intelligence with traditional MRI scanning.
"Prostate cancer screening and detection methods adoption has changed little over the past 30 years, despite the mountain of evidence pointing to the efficacy of superior technologies and the futility of the old methods," says the company founder and CEO Dr. Randall W. Jones. "Sadly, this has resulted in the unnecessary and premature deaths of countless numbers of men in the US alone. ProstatID represents an exciting step in the fight to save lives."
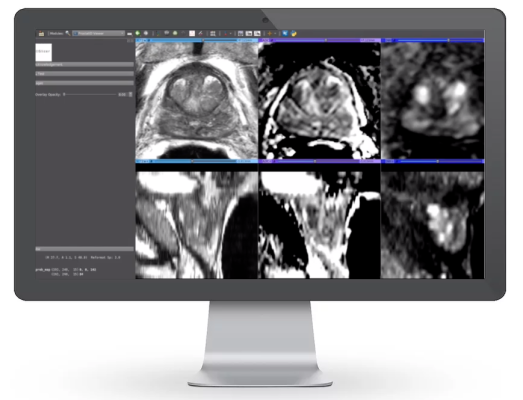
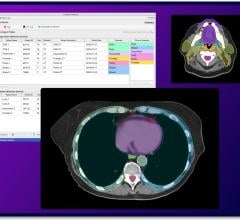
Trained by analyzing thousands of MRI image sets, radiological interpretations, guided biopsies, and pathology lab results, the software's algorithm recognizes and measures the volume of the prostate gland, and detects suspicious cancerous lesions - assigning a cancer probability to each one, and suggests a diagnostic case score known as PI-RADS (Prostate Imaging Reporting and Data Sytem) score. This was proven in two clinical studies (involving 25 radiologists from around the US) to significantly improve radiologic interpretation accuracy as measured by improved detection and fewer false positives when radiologists used the aid of the CAD. This demonstrates a tremendous savings of time and cost for the patient and provider, potentially saving lives in the process via early and accurate detection.
ProstatID's ability to detect lesions and assign a cancer probability to prostate MRI cases goes far beyond existing technologies which have improved on-screen formatting of prostate MRI cases and segmentations of a patient's prostate but stopped short of aiding in detection and diagnosis.
The computer-aided design tool is currently available for use as a Software-as-a-service (SaaS) device which requires only a secure VPN tunnel connection between the radiology department server or MRI system and the cloud-based ProstatID server. The software and connectivity are both HIPAA compliant and cyber-secure.
"With FDA clearance, the path for implementation is open", says Jones, "and hospitals and radiological clinics can connect in as little as one hour of IT effort, and begin bringing this exciting technology to their patients".
For more information: https://www.botimageai.com/

 May 02, 2024
May 02, 2024