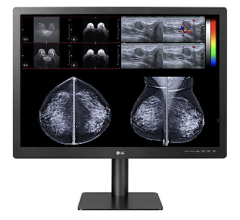
May 19, 2011 - Barco has launched the Mammo Tomosynthesis 5 MP, the first display system that has been cleared by the U.S. Food and Drug Administration (FDA) for breast tomosynthesis. The display comes with some groundbreaking technologies specifically developed for multi-modality mammography.
The Mammo Tomosynthesis 5MP efficiently displays high-quality multi-modality images. Barco's RapidFrame technology speeds pixel refresh when reviewing multi-frame image sequences such as tomosynthesis or breast MRI, eliminating blurring or ghosting. Combined with Barco's Per Pixel Uniformity (PPU) to remove disturbing screen noise, the display renders mammograms with the best image quality, helping radiologists make accurate diagnoses while speeding up their workflow.
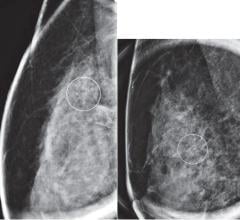
Equipped with the powerful DuraLight Nova backlight, the Mammo Tomosynthesis display offers twice the calibrated brightness (1,000 cd/m²) of conventional displays, while more than doubling the lamp lifetime (50,000 hours). This luminance enables radiologists to see fine details in dense breast tissue and renders near skin-line details more clearly. The display also provides an I-Luminate “hot light” button for extra brightness, which can reveal subtle details, even in dark areas.
The Mammo Tomosynthesis 5MP comes with an adjustable dual-head stand and allows users to angle the displays to their preferred position for optimum viewing. Additionally, the display can be viewed from a wide angle, without sacrificing contrast or black level. Images can now be read from any seat at the reading station, allowing radiologists to discuss the images with colleagues in the room or use them for educational purposes.
The display system's intrinsic brightness reduces eye fatigue and enables reading in a more pleasant ambient room light, preserving visibility of low-contrast details. It also includes the integrated I-Guard sensor, extra-large knee Array coil for the Vantage Titan MR system, which automatically checks contrast and luminance. This provides radiologists with an optimal work environment, boosting diagnostic performance and productivity.
For more information: www.barco.com

 May 01, 2024
May 01, 2024