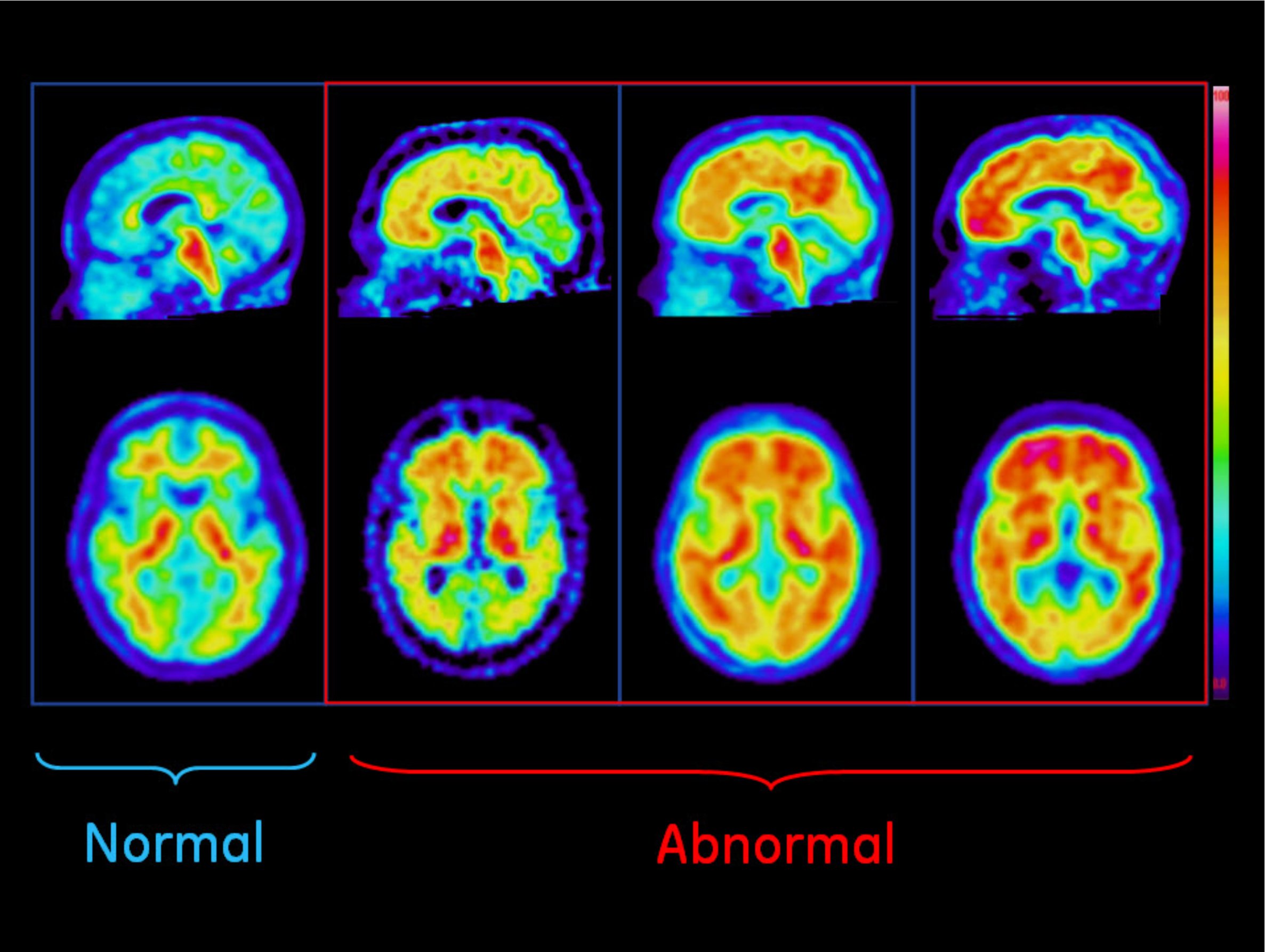
April 13, 2012 — GE Healthcare announced the preliminary results of two Phase 3 studies of its investigational positron emission tomography (PET) amyloid imaging agent, F-18 flutemetamol, where both studies met their primary endpoints. One study, in terminally ill patients who agreed to undergo brain autopsy, showed strong concordance between flutemetamol PET images and Alzheimer’s disease-associated beta amyloid brain pathology. The other study, in young healthy volunteers under age 40, had results concordant with the known lack of brain amyloid in this population. Full results of these studies will be presented in the coming months.
“The ability to detect or exclude significant amyloid deposits in the brain, along with other diagnostic tests, may help physicians make a more accurate assessment of patients with suspected Alzheimer’s disease (AD) and other cognitive disorders. The results from these studies are quite encouraging in demonstrating the potential of flutemetamol in that regard,” said Carl Sadowsky, M.D., clinical professor of neurology at Nova Southeastern University, Ft. Lauderdale, Fla. “We need an accurate diagnosis and better treatment, as accurate diagnosis has the potential to enable better patient management, and may also save cost.”
The accumulation of beta amyloid in the brain is believed to play a role leading up to the degeneration of neurons in AD and is one of several pathological characteristics implicated in the development of AD. Currently, AD is confirmed by histopathological identification of core features, including beta amyloid plaques, in post-mortem brain samples. Targeted amyloid imaging agents are being studied to determine their ability to help physicians detect amyloid deposition in living humans.
“Flutemetamol may well prove to be a clinically valuable component of a broader diagnostic workup that neurologists use when assessing patients with cognitive impairments, who may have AD,” said Jonathan Allis, MI (molecular imaging) PET segment leader, GE Healthcare Medical Diagnostics. “These studies support our application for regulatory approval of flutemetamol, and we intend to file later this year.”
Flutemetamol is a GE Healthcare PET imaging agent in development for the detection of beta amyloid, and is part of a portfolio of diagnostic solutions the company is currently developing in the Alzheimer’s field. GE Healthcare is taking a comprehensive approach to understanding AD through its ongoing research to uncover the causes, risks and physical effects of the disease. For example, the company is partnering with pharma to identify a biosignature, or a biological indicator, which may help physicians diagnose AD prior to the onset of clinical symptoms.
Earlier this week, the U.S. Food and Drug Administration (FDA) approved the first diagnostic agent to image Alzheimer’s disease beta-amyloid neuritic plaques in the living brain. Eli Lilly and Company and Avid Radiopharmaceuticals Inc., a wholly owned subsidiary of Lilly, released florbetapir (Amyvid), a positron emission tomography (PET) radioactive tracer agent indicated for brain imaging of beta-amyloid plaques in patients with cognitive impairment who are being evaluated for Alzheimer's disease and other causes of cognitive decline.
For more information: www.gehealthcare.com


 April 05, 2024
April 05, 2024