Breast density notification is an ongoing topic that gained a lot of momentum in 2015, and continues to do so in 2016. Not surprisingly, many of the sessions and studies presented at the 2015 meeting of the Radiological Society of North America (RSNA) in December focused on density issues.
Some History
According to Dense Breast Info, an educational coalition, 40 percent of women 40 and over have dense breasts, and cancer risk can be four to six times greater in women with extremely dense breasts than in women with low breast density. Although mammograms find some cancers not seen on other screening tests, in dense breasts, mammograms will miss more than 50 percent of the cancers present. It is clear that more research is needed on breast density in order to better understand its full impact on breast health.
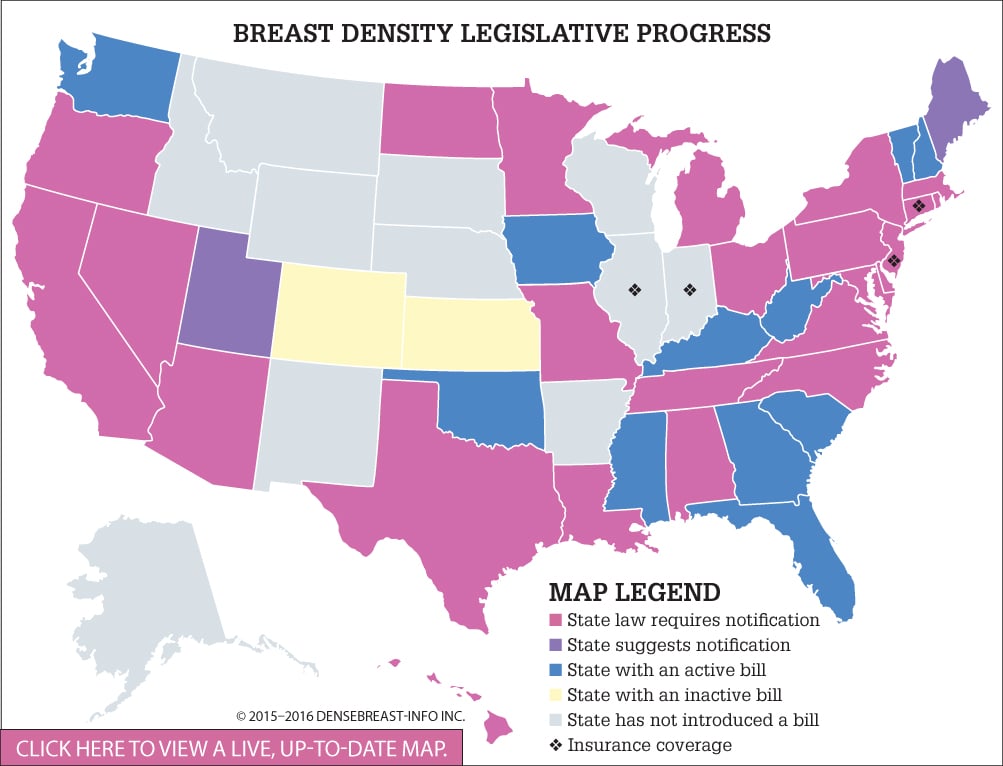
To date, 24 U.S. states have adopted legislation requiring healthcare providers to notify women whose mammograms reveal dense fibroglandular breast tissue. To track the progress of legislation across the country, DenseBreast-info.org has created a color-coded map separating states into six categories: States with a law requiring notification on the books (24); states that suggest notification (2); states with a 2015 or 2016 bill (11); states with an inactive bill (2); states with no active bill (15); and states that offer some level of insurance coverage related to breast density screening (4). Federal legislative efforts are ongoing. (See Breast Density Legislative Progress.)
A new study released at RSNA suggests that breast density alone is not a risk factor for cancer. In this study, researchers looked at data from 52,962 mammography exams done on women 50 to 69 over a five-year time period.
“In our study, we found that there was no significant difference in breast density between breast cancer patients and the control group in the screening program,” stated Natasa Katavic, M.D., Department of Radiology at Health Center Osijek in Osijek, Croatia, and co-author of the study. “We wanted to find out if breast cancer patients had more dense breast tissue than the healthy women. Also, we wanted to see what the percentage of dense breasts was in our postmenopausal population and, consequently, determine the value of mammography screening for this group.”
The study concluded that of 230 detected breast cancers, nearly half were from the group with the lowest category of breast density, while slightly less than 3 percent came from women in the highest breast density category. It did not find a strong association between higher mammographic densities and a higher risk of breast cancer among postmenopausal women. “Our study suggests that breast density alone might not be a strong independent risk factor for breast cancer. In risk assessment, all risk factors should be considered before decisions on additional examinations,” said Katavic.
Trends in Reporting
In other research presented at RSNA, Manisha Bahl, M.D., M.Ph., breast imaging fellow at Duke University Medical Center, discussed how breast density notification laws have had an immediate, but not a long-term, impact on dense breast reporting, however recognizing that they have brought renewed attention to the topic and importance of breast density. The purpose of this study was to evaluate trends in the reporting of breast density in response to breast density notification legislation.
This study used the American College of Radiology’s National Mammography Database (NMD). Researchers collected state-level data, monthly over a 20-month period, on the percentage of mammograms reported as heterogeneously dense or extremely dense and the breast cancer detection rate. Z-tests were used to calculate differences in proportions, and p-values less than 0.05 were considered statistically significant. A Z-test is a hypothesis test based on the Z-statistic, which follows the standard normal distribution under the null hypothesis.
“Our results suggest that radiologists downgraded breast density assessment immediately after law enactment, but then resumed pre-law reporting patterns shortly thereafter,” she said.
She cited two possible explanations for this: Radiologists may just want to avoid the new requirements for reporting, or they downgraded assessments fearing an influx in women wanting supplemental screening.
The NMD database includes data from 4 million mammograms spanning from 2010 to 2014, with results showing a statistically significant decrease in the percentage of mammograms reported as dense in the month after enactment of the law, compared to the prior month. She concluded that as a result, it appears the legislation was the catalyst for the decrease, noting the fluctuations did not occur in states without the law during that same time period.
Inconsistent Density Assignment
Recently, researchers at UC Davis and other institutions released a study stating that more research is needed on the evaluation of dense breasts. Their research found that the determinations of breast density can be unreliable, and that as many as 19 percent of women are re-categorized as dense rather than non-dense or vice versa from one mammogram to the next. The study also found that supplemental diagnostic screenings for women with dense breasts helps to find additional breast cancers, but also greatly increases false positive results.
The review of 24 studies among 2,067 citations examined the evidence on the consistency of breast density category assignment and on supplemental screening beyond standard mammography for women with dense breasts. “The number of states mandating that breast density information be reported to patients is increasing,” said Joy Melnikow, director of the Center for Healthcare Policy and Research at UC Davis and first author on the paper. “But the assignment of breast density is not terribly consistent. The policy may be out in front of the science.”
Breast density has been categorized through the Breast Imaging Reporting and Data System (BI-RADS). Breasts are defined as: a) mostly fatty, b) scattered density, c) consistent density and d) extremely dense. Higher breast density modestly increases a woman’s risk of developing breast cancer and decreases the sensitivity and specificity of screening mammograms. Women classified with dense breasts (categories c and d) may be offered supplemental screening with ultrasound or magnetic resonance imaging (MRI). However, the study shows this approach may not be supported by strong scientific evidence, citing that one problem with this reporting is the inability to accurately assess if a woman even has dense breasts.
“It is important to be clear who actually has dense breasts,” said Melnikow. “Also, when patients are told their breasts are either dense or not dense, they need to have confidence in that assessment. It’s clear from our review of ultrasound and MRI that if you go beyond mammography to try to find breast cancers you can find them, but whether there’s a benefit to women’s health from looking is not established. At this point, there are no studies on clinical outcomes.”
Melnikow believes the best solution for these problems is to conduct long-term, rigorous research to better standardize breast density classification and determine whether supplemental screenings provide actual health benefits for women. The U.S. Preventive Services Task Force (USPSTF) found insufficient evidence to assess the balance of benefits and harms for supplemental screening of women with dense breasts with ultrasound, MRI or other modalities. The USPSTF recommendations continue to be an ongoing controversy that undoubtedly will remain in 2016.
(See USPSTF Final Recommendations.)
“It’s important that policies come from the evidence,” said Melnikow. “It’s also important that women not overreact to information about their breast density.”
Sidebar:
USPSTF Final Recommendations
The U.S. Preventive Services Task Force (USPSTF) just released its final recommendation statement on screening for breast cancer following an in-depth review of the science on the benefits and harms of screening mammography, and a detailed review of input received from the public and healthcare professionals on its 2015 draft recommendation. The Task Force is an independent, volunteer panel of experts in evidence-based medicine.
Here are the highlights of the statement:
• The Task Force confirmed that screening mammography is effective in reducing deaths due to breast cancer in women 40-74. The greatest benefit, according to USPSTF, is in women 50-74, with the best balance of benefits to
harm when screening is done every two years. (B recommendation)
• For women in their 40s, mammography screening every two years can also be effective. USPSTF recommends that the decision to start screening should be an individual one, taking into account health history, preferences and the value of potential benefits versus harm.
(C recommendation)
• The likelihood of benefit is less for older women, and the potential harm is proportionally greater. The most serious potential harm is unneeded treatment for a type of cancer that would not have become a threat to health over the lifetime. Another harm is a false-positive test result that can lead to additional tests and procedures, anxiety and stress.
The Task Force also identified several areas where additional research is needed to better understand the benefits of screening. It concluded that evidence is insufficient to determine the balance of benefits and harm in three areas: the benefits and harm of screening women 75 and older; adjunctive screening in women with dense breasts; and the effectiveness of 3-D mammography for the detection of breast cancer. The Task Force strongly encourages additional research in these areas and notes that women should speak with their doctors to determine what is best for their individual needs.


 April 18, 2024
April 18, 2024 








