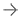Ascelia Pharma recently presented data from its Orviglance Hepatic Impairment study showing that Orviglance, a non-gadolinium oral contrast agent, has the potential to be used in patients with any degree of liver impairment. The data was presented during the June 14-16 European Society of Gastrointestinal and Abdominal Radiology (ESGAR), held in Valencia, Spain. Image courtesy: Ascelia Pharma
June 20, 2023 — Ascelia Pharma presented data from its Orviglance Hepatic Impairment study during the European Society of Gastrointestinal and Abdominal Radiology (ESGAR) 2023 annual meeting held June 14-16 in Valencia, Spain. The data from the study shows that Orviglance, a non-gadolinium oral contrast agent, has the potential to be used in patients with any degree of liver impairment.
Ascelia Pharma is a clinical stage biopharmaceutical company focused on developing orphan oncology treatments to address unmet medical needs. Orviglance can be used in MRI scans to improve detection and visualization of focal liver lesions in patients with severely impaired kidney function who, due to the risk of serious side effects, cannot use the currently available contrast agents.
The results from the Hepatic Impairment Study were presented with a poster titled "Safety and signal intensity of a novel liver-specific MRI contrast agent," Orviglance (manganese chloride tetrahydrate), in adult subjects with mild, moderate, or severe hepatic impairment. The study was performed at the Texas Liver Institute in the US in patients with hepatic impairment defined by the Child-Pugh score. The volunteers were divided into three severity groups, each of which had 6 participants, who were matched to a control group with normal hepatic function.
The results of the Hepatic Impairment Study showed that Orviglance is well tolerated in patients with liver (hepatic) impairment, with only mild to moderate transient, gastrointestinal adverse events reported, such as nausea. No new safety concerns were identified. The data confirmed there was no renal excretion of Orviglance, and that excretion is primarily occurring via the liver also in this subgroup of patients. The data suggests that Orviglance can be used in patients with any degree of hepatic impairment.
Orviglance, which has been granted an Orphan Drug Designation by the US Food and Drug Administration (FDA), is the first contrast agent in development for use in liver magnetic resonance imaging (MRI) in patients with severely impaired kidney function. Ascelia Pharma demonstrates that Orviglance improves the detection and visualization of focal liver lesions, including liver metastases and primary tumors, without causing a risk to patients. A clinical program of nine studies, including the global Phase 3 study SPARKLE, have been completed.
The biotech company, focused on orphan oncology treatments, also hosted Q&A session with two panelists to discuss liver imaging and the unmet need for patients with severe kidney impairment. The key experts were: Dr. Nikolaos Kartalis of the Department of Radiology Huddinge, Karolinska University Hospital, and Alessandro Furlan, MD, of the Radiology, Abdominal Imaging Division of University of Pittsburgh Medical Center and Pittsburgh Liver Research Center.
In the session, moderated by Director of Global Medical Affairs at Ascelia Pharma, Dr. Nadilka Hettiarachchige, Drs. Kartalis and Furlan discussed the role of liver imaging in cancer care, the clinical practice for patients with severe kidney impairment and the trends ahead.
“With the improvement of the treatment and introduction of new treatment methods… we have a tendency to see these patients quite often. So, we follow these patients; we see them many times,” said Kartalis.
“It’s not infrequent to find patients that, on top of having metastatic disease, also have impaired renal function,” said Furlan. He added: “In my institution, for patients that have reduced renal function, we would reduce the amount of gadolinium that we inject, since we still do not have an alternative — a non-gadolinium-based contrast agent that we can use,” and continued “we don’t have that yet in the market, but it would certainly be a great tool to have in our box.”
“We are honored to have been given the opportunity to chair this debate on liver imaging and unmet needs for patients with two of the leading experts in liver imaging and to share with the medical community that Orviglance has the potential to also be used in patients with any degree of hepatic impairment,” said Julie Waras Brogren, Deputy CEO and CCO of Ascelia Pharma, whose global headquarters are in Malmö, Sweden. Brogen also noted that the company is on track for announcing results from SPARKLE, the pivotal Phase 3 study for Orviglance, during the middle of this year.
For more information: www.ascelia.com
Related coverage:
Study compares effect of food intake on manganese-based MRI contrast agent absorption


 May 29, 2024
May 29, 2024