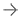
January 16, 2019 — MIM Software Inc. received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for molecular radiotherapy (MRT) dosimetry.
MRT is an effective form of therapy that uses radiopharmaceuticals such as Lutathera (Lu-177 DOTATATE) and Azedra (I-131 iobenguane) to target tumors based on certain receptors that these tumors express.
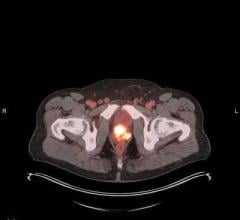
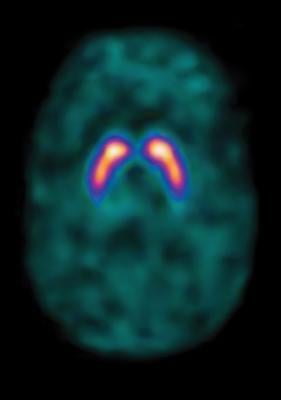
Until now, there has not been an effective way to measure the absorbed dose from MRT for an individual patient. This is due to a lack of access to quantitative single photon emission computed tomography (SPECT) images and tools for calculating dose on the patient's own anatomy. MIM SurePlan MRT provides both quantitative SPECT reconstruction and voxel-based absorbed dose calculation by utilizing the patient's own images, allowing for personalized dosimetry measurements.
MIM SurePlan MRT provides timesaving tools for organ and tumor segmentation, deformable registration and voxel-based dosimetry for molecular radiotherapy. Other features of MIM SurePlan MRT include multi-tracer theranostics support, quantitative SPECT and planar corrections, and dosimetry reporting tools.
MIM Software will demonstrate MIM SurePlan MRT at the Society for Nuclear Medicine and Molecular Imaging (SNMMI) 2019 Mid-Winter Meeting, Jan. 18-19 in Palm Springs, Calif.
For more information: www.mimsoftware.com


 May 17, 2024
May 17, 2024