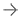
HALO AI now includes the option of three powerful neural networks – VGG, DenseNet and MiniNet. VGG, a well known and more traditional network, was used to build the Indica Labs submission in the CAMELYON17 challenge and was the first neural network integrated with HALO AI.
February 14, 2023 — Indica Labs, an industry leader in quantitative digital pathology and image management solutions, and Hamamatsu Photonics K.K., a forerunner in whole slide imaging systems, announced an agreement to maintain long-term interoperability between Indica Lab’s HALO AP software platform and Hamamatsu’s NanoZoomer family of high-speed, high-resolution scanners.
Indica Lab's browser-based, CE-marked* HALO AP software brings case-centric digital pathology slide management, collaboration, and AI-powered quantitative image analysis to Anatomic Pathology labs worldwide. In keeping with Indica Lab's ethos of an accessible and open pathology ecosystem, HALO AP is scanner agnostic and integrates with a variety of third-party software applications, including AI solutions and LIS/LIMS platforms, using flexible, easy-to-use APIs and software development kit (SDK).
Hamamatsu brings over 60 years of photonics engineering to bear on the development of NanoZoomer scanners, resulting in instruments that are renowned for image quality, reliability, and ease of use. When coupled with Hamamatsu’s dedication to customer service, Hamamatsu users experience the best combination of robust performance and personalized support.
As laboratories adopt digital solutions, the challenge for laboratory managers is to integrate an array of hardware and software and achieve maximum efficiency. This collaboration between Indica Labs and Hamamatsu streamlines this process.
"Providing interoperability and scalable solutions is critical to the adoption of digital pathology." commented Don Ariyakumar, NanoZoomer Product Manager at Hamamatsu. "We believe that customers should be able to choose best-of-class products. The option to implement the most appropriate hardware and software for their specific needs increases the value of digital pathology for all stakeholders."
Hamamatsu's technical collaboration with Indica Labs will facilitate Indica Labs approach to regulatory clearances utilizing the HALO AP system in conjunction with the NanoZoomer S360MD* for primary diagnosis. Ongoing technical performance assessments to verify that Hamamatsu’s image format is accurately displayed in all Indica Labs’ viewers guarantees continued interoperability of Indica and Hamamatsu products and will help drive the adoption of these technologies within the pathology community.
Indica Labs' CEO, Steven Hashagen commented "This agreement is an important reassurance to our mutual customers that both companies are committed to working together on technical, regulatory, and commercial fronts. It ensures we can achieve image fidelity and interoperability across our viewing and analysis software, allows us to align our regulatory submissions, and facilitates sales and support cooperation for our mutual customers as we have demonstrated in the past."
The relationship between Indica Labs and Hamamatsu resulted in the win of significant digital pathology tenders in the United Kingdom, most notably Nottingham University Hospitals (NUH) NHS Trust. NUH is the third largest NHS Trust in the United Kingdom, serving over 2.5 million patients, and generating 340,000 pathology slides per year. The goal of the NUH implementation is full digitization of the pathology workflow. Read further details of the NUH NHS Trust HALO AP and NanoZoomer implementation here https://indicalab.com/news/press-release-nuh-nhs-trust-selects-indica-labs-halo-ap-as-digital-pathology-solution/.
For more information: https://indicalab.com/


 May 17, 2024
May 17, 2024