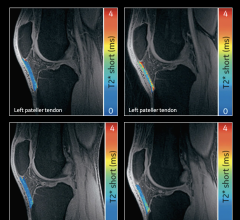
September 8, 2017 — Delphinus Medical Technologies Inc. announced that the SoftVue Discover Breast Ultrasound Prospective Case Collection project has launched, enrolling the first patient. Qualified participants will undergo screening digital mammography as well as SoftVue 3D whole breast ultrasound exams. Information gathered from the project will compare SoftVue imaging to digital mammography to determine the effectiveness of detecting additional cancers with SoftVue that are not seen with mammography alone, particularly in women with dense breast tissue.
The project will enroll 10,000 asymptomatic women with dense breast tissue at several centers in the United States. More than 40 percent1 of women nationwide have dense breast tissue, which is unrelated to weight or breast size. Because dense breasts can mask potential cancers on mammography, the sensitivity for detecting breast cancer is lower in women with dense breasts. Studies have shown that in dense breasts, ultrasound can detect cancers not seen on mammography. However, traditional handheld ultrasound exams can be time-consuming and often operator-dependent. They also have a high rate of false positives, resulting in unnecessary biopsies and added cost to the healthcare system.
In contrast, SoftVue is a fast, fully automated and gentle system that conducts scans while a woman lies on her stomach on a padded table, with her breast supported in a warm water bath. A 360-degree ring transducer images the entire breast in a single pass, moving from the front of the breast to the chest wall. The entire scan takes about two to four minutes per breast, and there is no radiation exposure or compression of the breast. Unlike handheld ultrasound, SoftVue can provide multiple distinctive tissue qualities to radiologists, allowing them to differentiate possible cancers from normal to benign findings.
“Dense breast tissue can mask or hide cancer, making it more difficult for mammography to detect cancer. And while ultrasound has been shown to be effective in detecting cancer in dense breasts, there’s a need for advanced technology like SoftVue that enables fast and comfortable whole breast ultrasound with fewer false positives," said Mary Yamashita, M.D., assistant professor of clinical radiology at the University of Southern California (USC) Norris Comprehensive Cancer Center and the national principal investigator of the Discover Breast Ultrasound project. “This important research will help us confirm the efficacy of the SoftVue system and potentially help establish new standards of care in dense breast tissue cancer screening.”
The Discover Breast Ultrasound project is recruiting participants at centers that include:
- USC Norris Comprehensive Cancer Center;
- Mt. Sinai Medical Center;
- Beaumont Hospital - Dearborn;
- Elizabeth Wende Breast Care;
- Southcoast Health Imaging;
- St. Elizabeth Hospital, part of Ascension; and
- Weinstein Imaging.
Data from this study will support the company’s submission of a premarket approval (PMA) application to the U.S. Food and Drug Administration (FDA) for a supplemental screening indication for women with dense breasts in combination with mammography. SoftVue has already received two 510(k) clearances from the FDA for diagnostic breast ultrasound imaging and is not intended for use as a replacement for screening mammography. The technology is currently only in use at participating sites.
For more information: www.discoversoftvue.com
References
1. Sprague BL, et al. Prevalence of Mammographically Dense Breasts in the United States. J Natl Cancer Inst. 2014 Oct; 106(10): dju255. Published online 2014 Sep 12.


 May 28, 2024
May 28, 2024