June 12, 2025 — GE HealthCare has announced the combination of GE HealthCare’s proprietary features and algorithms with MIM Encore, marking a significant milestone in its mission to deliver precision care through advanced digital solutions. This implementation brings powerful new features to
May 6, 2025 — NewVue.ai and MD.ai have announced an integration partnership that brings MD.ai’s structured reporting ...
June 12, 2025 — According to a new report published in the American Society of Radiation Oncology journal, Practical ...
June 12, 2025 — GE HealthCare has announced the combination of GE HealthCare’s proprietary features and algorithms with ...
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
June 11, 2025 — Diagnostic laboratory leaders view digital pathology and artificial intelligence (AI) as pivotal to ...
June 11, 2025 — To prepare healthcare workforces and providers for an AI-driven future, Qure.ai has expanded its Global ...
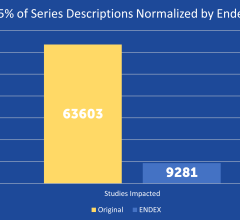
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
June 10, 2025 — CIVIE has announced the official launch of RadPod, an AI-driven, on-demand radiology platform designed ...
While most women understand the importance of health screenings, an estimated 72 million have missed or postponed a ...
June 4, 2025 — RadNet, Inc., a provider of high-quality, cost-effective diagnostic imaging services and digital health ...
June 9, 2025 — A new independent, peer-reviewed study published in the journal Clinical Breast Cancer reinforces the ...
May 27, 2025 — A new study from Mount Sinai's Department of Radiation Oncology reveals that most new radiation ...
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
June 5, 2025 — Hand-blown x-ray tubes dating back to 1897, a 1916 mechanical rectifier that delivered a high-voltage ...
As scientists working in a range of life science and applied industrial applications seek deeper insights into complex ...
June 05, 2025 — Nano-X Imaging Ltd. has announced that its deep-learning medical imaging analytics subsidiary, Nanox AI ...
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
June 5, 2025 – Artera, the developer of multimodal artificial intelligence (MMAI)-based prognostic and predictive cancer ...
June 3, 2025 — In a collaborative study between the Departments of Radiology at the Children’s Hospital of Philadelphia ...
June 2, 2025 — Clairity, Inc., a digital health innovator advancing AI-driven healthcare solutions, has received U.S ...
May 30, 2025 — GE HealthCare recently announced that the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) ...
Hyperfine, Inc., producer of the world’s first FDA-cleared AI-powered portable MRI system for the brain — the Swoop ...
May 27, 2025 — DeepLook Medical, a company advancing medical imaging through visual enhancement technology, recently ...
May 20, 2025 — Royal Philips has launched the RADIQAL (Radiation Dose and Image Quality Trial) trial. This multicenter ...
May 20, 2025 – Intelerad, a provider of medical imaging software solutions, recently announced its prime partnership ...







 June 12, 2025
June 12, 2025 






















