May 20, 2025 — Royal Philips has launched the RADIQAL (Radiation Dose and Image Quality Trial) trial. This multicenter, randomized study, sponsored by Philips will enroll 824 coronary artery disease patients across 6 hospitals in Spain, Czech Republic and the US. The first patient in the study was
May 27, 2025 — A new study from Mount Sinai's Department of Radiation Oncology reveals that most new radiation ...
June 5, 2025 — Hand-blown x-ray tubes dating back to 1897, a 1916 mechanical rectifier that delivered a high-voltage ...
As scientists working in a range of life science and applied industrial applications seek deeper insights into complex ...
eHealth Saskatchewan plays a vital role in providing IT services to patients, health care providers, and partners such ...
June 05, 2025 — Nano-X Imaging Ltd. has announced that its deep-learning medical imaging analytics subsidiary, Nanox AI ...
June 5, 2025 – Artera, the developer of multimodal artificial intelligence (MMAI)-based prognostic and predictive cancer ...
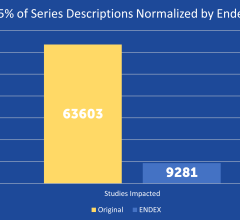
SPONSORED CONTENT — EnsightTM 2.0 is the newest version of Enlitic’s data standardization software framework. Ensight is ...
June 3, 2025 — In a collaborative study between the Departments of Radiology at the Children’s Hospital of Philadelphia ...
While most women understand the importance of health screenings, an estimated 72 million have missed or postponed a ...
June 2, 2025 — Clairity, Inc., a digital health innovator advancing AI-driven healthcare solutions, has received U.S ...
May 30, 2025 — GE HealthCare recently announced that the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) ...
Hyperfine, Inc., producer of the world’s first FDA-cleared AI-powered portable MRI system for the brain — the Swoop ...
Fujifilm’s APERTO Lucent is a 0.4T mid-field, open MRI system addressing today’s capability and image quality needs ...
May 27, 2025 — DeepLook Medical, a company advancing medical imaging through visual enhancement technology, recently ...
May 20, 2025 — Royal Philips has launched the RADIQAL (Radiation Dose and Image Quality Trial) trial. This multicenter ...
May 20, 2025 – Intelerad, a provider of medical imaging software solutions, recently announced its prime partnership ...
SPONSORED CONTENT — Fujifilm’s latest CT technology brings exceptional image quality to a compact and user- and patient ...
May 21, 2025 — Konica Minolta Healthcare Americas, Inc and NewVue have announced the introduction of Exa Teleradiology ...
May 13, 2025-- GE HealthCare recently announced the U.S. Food and Drug Administration (FDA) has approved a pediatric ...
May 19, 2025 - Arineta, a provider of cardiovascular imaging solutions, recently announced that its SpotLight Duo ...
May 15, 2025 – Royal Philips, a global leader in health technology, has released its 10th annual Future Health Index ...
May 14, 2025 – Bialogics Analytics Inc., a leader in radiology informatics, has introduced its new AI solution AI ...
May 13, 2025 — In one of the larger studies of its kind, researchers have identified six breast texture patterns that ...
May 15, 2025 — GE HealthCare has launched CleaRecon DL, technology powered by a deep-learning algorithm, to improve the ...
May 14, 2025 — Siemens Healthineers is investing $150 million in new projects to expand production, create jobs and ...







 June 06, 2025
June 06, 2025 






















