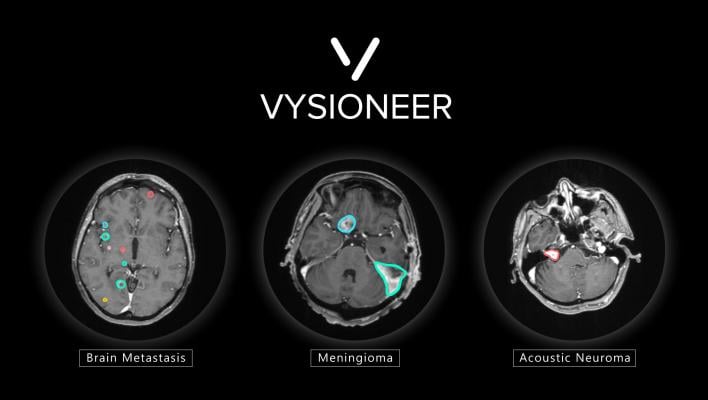
Brain tumors edged out by artificial intelligence: VBrain applies auto-contouring to the three most common types of brain tumors: brain metastasis, meningioma and acoustic neuroma.
April 7, 2021 — Vysioneer, a leader in artificial intelligence (AI) for cancer care, announced it received the U.S. Food and Drug Administration (FDA) clearance for VBrain, the first-ever AI-powered tumor auto-contouring solution.
VBrain is a game changer for radiotherapy treatment planning, enabling a quicker response time for performing radiation therapy with more precision in targeting the tumor. Manual contouring time that may take several hours by clinicians is completed within a few minutes with the assistance of VBrain. As the first of its kind, the fully automated solution also ensures precision mapping of brain tumors with closer cuts and the ability to identify additional lesions that may be missed by the human eye. This is the first time the FDA has cleared an AI device for tumor auto-contouring in radiation therapy. Devices to receive FDA clearance prior to VBrain are specific to normal organ auto-contouring.
VBrain was tested at multiple sites throughout the U.S. and Taiwan before FDA approval. The solution is cleared to apply auto-contouring to the three most common types of brain tumors: brain metastasis, meningioma and acoustic neuroma. Additionally, the solution was extensively and rigorously tested through an 18-month clinical integration at National Taiwan University Hospital (NTUH), a leader in medical care for the country, with results published on the world's leading medical journal, Neuro-Oncology.
Clinical findings indicated clinicians assisted by VBrain demonstrated 12.2 percent higher sensitivity for lesion detection, and less experienced clinicians improved contouring accuracy with the added help. The efficiency in AI assistance also decreased treatment planning time at a median of 30.8 percent. "There were distinct accuracy and efficiency improvements for clinicians of all skill levels," said Jason Chia-Hsien Cheng, M.D., FASTRO, former director of radiation oncology at NTUH. "VBrain has a unique opportunity to influence future treatment on a global scale as a cloud-based software. Clinicians around the world, including areas lacking in resources, could utilize VBrain to achieve the world class standard of contouring."
VBrain has been integrated through Vysioneer's partnership with Mary Bird Perkins Cancer Center, a cancer care organization headquartered in Baton Rouge, Louisiana. Collaboration with a leading cancer center will allow authentication of the clinical and financial benefits offered by VBrain.
"I am thrilled to bring VBrain to our partners across the U.S. and Taiwan," said Jen-Tang Lu, CEO of Vysioneer. "Receiving unique FDA clearance for this solution allows Vysioneer to further its commitment of transforming radiotherapy workflows through developing full body auto-contouring solutions. The future of AI is near, bringing a second set of eyes and hands to assist clinicians in analyzing and segmenting medical scans and further improving patient cancer care."
For more information: www.vysioneer.com


 May 17, 2024
May 17, 2024