September 15, 2020 — Varian announced it has received FDA 510(k) clearance for its Eclipse v16.1 treatment planning software for proton therapy. Proton therapy is the most sophisticated radiotherapy technology available today, using protons, accelerated to about two-thirds the speed of light, or more than 100,000 miles per second, to destroy cancer cells, while minimizing exposure to nearby healthy tissue.
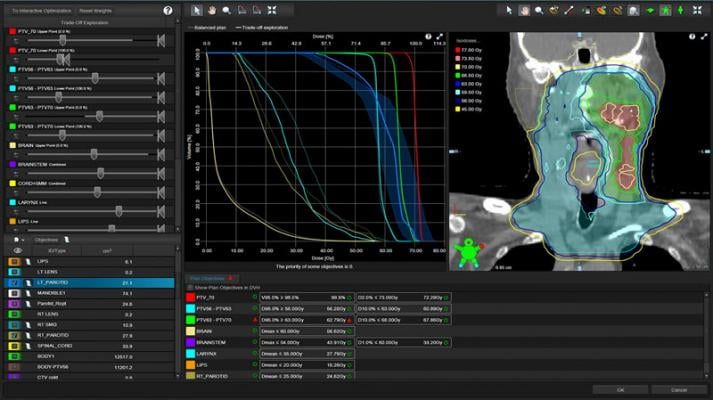
With the release of Eclipse v16.1 treatment planning software, Varian introduces intelligent treatment planning features, leveraging Siemens Healthineers' SOMATOM DECT diagnostic imager with its dual X-ray energies capability. This new capability not only enhances visibility of soft tissue, but also provides more accurate information about tissue density allowing dosimetrists to plan more precisely. This feature was validated through a clinical collaboration with the Roberts Proton Therapy Center at the University of Pennsylvania. Also included in Eclipse v16.1 is the first clinical release of GPU Monte Carlo proton dose calculation technology, that was evaluated in partnership with the Emory Proton Therapy Center in Atlanta, Georgia. This technology enables clinicians to accelerate calculation speed and improve overall treatment planning efficiency.
"As a leader in proton therapy, Varian's goal is to drive the next evolution of proton treatment planning by providing intelligent tools designed to improve plan quality, accelerate speed of dose calculations, and provide new levels of accuracy," said Kolleen Kennedy, President, Proton Solutions & Chief Growth Officer, Varian. "With the launch of Eclipse v16.1 we continue to build on our strong foundation, providing the most sophisticated proton therapy planning solutions to our clinical users across the globe."
For more information: www.varian.com/eclipse

 May 06, 2024
May 06, 2024