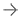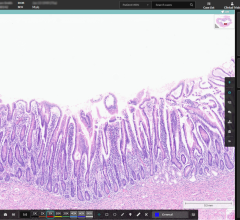
Cancer diagnotics company Paige has announced new advanced integration capabilities to the Paige Platform, a holistic web-based solution built for AI that enables complete digital pathology workflows and supports the evolving needs of modern labs. Image courtesy: Paige
May 18, 2023 — Paige, a global provider of end-to-end digital pathology solutions and clinical Artificial Intelligence (AI) applications to assist in diagnosing cancer, has introduced new advanced integration capabilities to the Paige Platform. In its May 18 announcement, the company noted that the platform is a holistic web-based solution enabling complete digital pathology workflows and supports the evolving needs of today’s modern lab. It further reported that the platform, clinically utilized by labs throughout the work, is the only digital pathology solution built for AI offering flexibility, scalability and customization.
With Paige’s flexible platform at the nexus of the digital pathology ecosystem, labs and pathologists can connect their existing and future digital pathology applications into a single environment managed by Paige. The Platform’s flexibility is powered by a set of APIs that allow for unlimited vendor-neutral integrations and enable custom workflows to be built on top of Paige’s already robust native capabilities. In addition to integrating within the workflow, the solution enables seamless access to both Paige’s AI solutions and third-party AI applications. This helps to support labs in taking an iterative approach to digital pathology adoption, reducing upfront investment, avoiding vendor lock-in and making the digital transition a frictionless experience for the entire pathology department.
Paige is the first company to receive FDA approval for a clinical AI application in digital pathology.
“Paige is the only AI-powered digital pathology platform being used daily all over the world that offers truly open, secure and scalable API capabilities. The Paige Platform drives diagnostic confidence and efficiency for pathologists in their quest to diagnose cancer quickly and accurately every single time,” said Andy Moye, Ph.D., Chief Executive Officer at Paige. “The open platform is thoughtfully designed to empower labs and pathologists to customize their workflows to their unique needs and leverage AI at all steps from case creation to reporting.”
The Paige Platform was developed for superior user experience and functionality, placing everything a digital pathologist may need at their fingertips, including diverse WSI scanner and LIS integration capabilities, a CE-IVD & UKCA marked and FDA-cleared whole slide image viewer, a digital case manager to help prioritize and streamline case review and immediate access to Paige and third-party AI applications. In the United States, European Union & United Kingdom, FullFocus (K201005) is cleared for clinical use with various scanner brands and models, including Philips Ultrafast, Leica AT2, Hamamatsu S60 and S360, and 3DHistech P1000 scanners. The company’s Platform was developed against the most secure standards in the industry, achieving ISO 27001 certification, and is also HIPAA and GDPR compliant.
For information: www.paige.ai
Related content:
FDA Authorizes Software That Can Help Identify Prostate Cancer


 May 16, 2024
May 16, 2024