July 13, 2015 - Mirada Medical and collaborators from the University Medical Center, Groningen, The Netherlands, will present new research on quality assurance of deformable image registration (DIR) at the annual meeting of the American Association of Medical Physicists (AAPM). Accurate definition of targets and critical structures is essential for the planning and delivery of radiation therapy and radiosurgery. DIR is increasingly utilized during treatment planning to aid in this process.
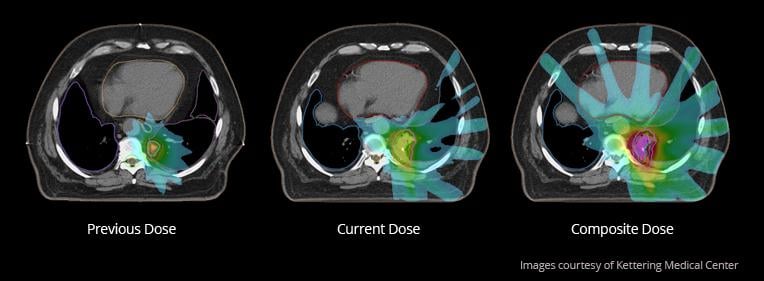
A critical step in deploying DIR in the clinic is quality assurance of the result. To date, this has been typically performed qualitatively using tools such as fusion overlays and inspection of the deformation field, requiring a user experienced with DIR, patient anatomy and the clinical context. Such an approach is subjective and can lead to significant inter-user variability. Existing quantitative tools such as measuring target registration error and dice overlap of structures are practical for commissioning and research but are often too labor-intensive for clinical use.
Mirada Medical and collaborators will be introducing RegistrationQX, a novel technique to overcome these limitations. RegistrationQX uses a set of quantitative measurements of the deformation quality, numerical robustness and matching to assist the user in assessing the quality of a DIR result. With the release of RegistrationQX, clinical staff will be able to augment their visual DIR quality assurance with an automatically generated quantitative report. The original research compares the quantitative scores produced by RegistrationQX with grading scores produced by experts with significant experience in using DIR clinically on 27 head and neck patients. Good correlation (0.86) was observed between the automatic and manual scores indicating that the quantitative scores reflected the opinions of the experts.
RegistrationQX and this study form part of Mirada's Registration Assurance program, a comprehensive program for ensuring DIR quality and continuous improvement through rigorous scientific validation of technology, education, comprehensive QC tools and a commitment to ongoing development and support. The registration workflows used in the study are all available in the new releases of Mirada RTx and Mirada Workflow Box.
For more information: www.mirada-medical.com


 May 17, 2024
May 17, 2024