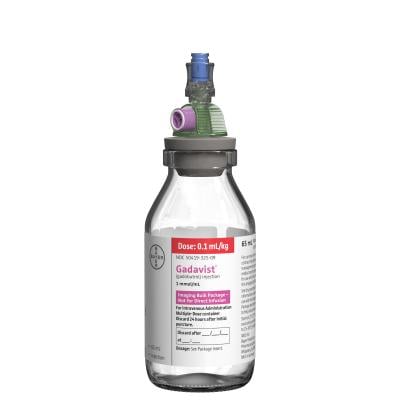
August 14, 2020 — The U.S. Food and Drug Administration (FDA) announced the approval of the Gadavist (gadobutrol) Imaging Bulk Package. The Gadavist Imaging Bulk Package will be available in two presentation sizes, 30 mL and 65 mL, and allows for weight-based dosing with a 24-hour stand time for multi-patient dosing.
In conjunction with the bulk package approval, the FDA also cleared the MEDRAD Imaging Bulk Package Transfer Spike that is indicated for the transfer of Gadavist contrast media as supplied in the Imaging Bulk Package presentation.
As the only MR contrast media FDA-approved Imaging Bulk Package for use with a cleared transfer spike, Gadavist Imaging Bulk Package offers key features to support the radiology workflow. After initial set up, the product can be used across multiple patients and work shifts for up to 24 hours after the first puncture of the Imaging Bulk Package. Compared to single-use bottles, the Imaging Bulk Package can potentially mean more complete usage of the contrast and less contrast and vial waste. In addition, technologists and nurses can prepare and administer weight-based dosing using a single multi-dose set up directly in the scan room. The bulk package adheres to the Joint Commission requirements for multi-patient dosing.1
“With the changing healthcare environment, Bayer is dedicated to investing not only in new innovations, but aligning our solutions to address some of the challenges that hospitals are facing today,” said Dennis Durmis, Head of Region Americas, Bayer Radiology. "We are focused on offering solutions in new areas of unmet need."
1 Sentinel Event Alert 52: Preventing infection from the misuse of vials. The Joint Comission web site. https://www.jointcommission.org/en/resources/patient-safety-topics/sentinel-event/sentinel-event-alert-newsletters/sentinel-event-alert-issue-52-preventing-infection-from-the-misuse-of-vials/. Accessed August 11, 2020.
For more information: www.bayer.com


 May 08, 2024
May 08, 2024