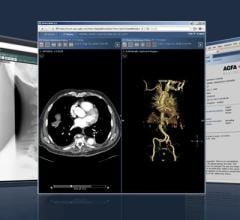
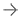Deciphex, a Dublin-based software company focused on developing digital pathology-based software and services for clinical and research pathology, has announced the rollout of its Automated Image Quality Control (QC) for digital research pathology. Photo credit: Deciphex
January 19, 2023 — In an effort to speed up the research study process, Deciphex has announced the rollout of its Automated Image Quality Control (QC) for digital research pathology. The software company, which focuses on developing digital pathology-based software and services for clinical and research pathology, noted the launch of Automated Image QC is a further step as part of its mission to integrate and enhance Pathologists’ capability to deliver their skills at scale.
By building Automated Image QC into Patholytix 3.0, Deciphex delivers dramatically faster turnarounds of studies, removing today’s common barriers and choke points in studies and development, according to a company update. It noted that histotechnicians spend a significant amount of time performing manual QC. This creates numerous downstream effects, including: pathologists must wait while slides are re-scanned; histotechnicians must re-scan, and review reworked slides, increasing their workload; study delivery times, and in turn, study completion times, are delayed; and delays at the preclinical stage of drug development lead to delays getting the drug to market
According to Dan Rudmann, DVM, PhD, Global Director of Digital Toxicologic Pathology at Charles River Laboratories, and global strategic partner of Deciphex, Patholytix eliminates the delays that have caused manual QC to put studies at risk. He offered this antecdote of a recent experience:
“The manual review of whole slide images (WSI) is slow and consistency is difficult. On the final day of study review for low and mid-dose animals for a recent study, I identified issues with a few WSI that required rescanning. The scanner was booked and the rescans were delayed by a day. This rework late in the primary diagnostic timeline put the study timelines at risk which is not something we want to do. We believe that an automated QC tool would not only increase the efficiency of the lab (less time for WSI review) but also mitigate the possibility of identifying WSI issues at the time of pathologist review.”
The Patholytix Automated Image QC module prechecks all study slides for blurring, striping and other artefacts, offering automatic selection of clear images for review, whereby whole slide images of good quality are sent to the pathologist for immediate review. Those that are of poor quality are flagged for manual review to determine if re-work is needed. For example, if 10% of slides in a study have QC issues, the tool can cut the histotech’s workload down by 90% as they would only need to manually review these slides.
Additional benefits of the automated Image QC tool are less false positives, user adjustable thresholds, and accelerated due diligence. Image QC automation will have a dramatic impact on drug development programs.
Deciphex offers a range of services for clinical and research pathology, including its Patholytix platform for research pathology, and its Diagnexia clinical service, which provides remote subspecialty pathology services. The company's solutions have already been adopted by leading research organizations and pharmaceutical companies, and its products are trusted by customers around the world.
As reported by Imaging Technology News, in December 2022, Deciphex introduced digital pathology review and scoring in a single platform for the first time, reporting that the research platform Patholytix 3.0 offered, for the first time, the ability of research pathologists to review and score non-clinical studies on a single platform, resulting in faster pathology reviews, with less risk of error.
For more information: www.deciphex.com
Related Content:
Deciphex Launches New Platform for Digital Pathology Review and Scoring
Deciphex Launches its Diagnostics as a Service (DxaaS) in US


 May 24, 2024
May 24, 2024