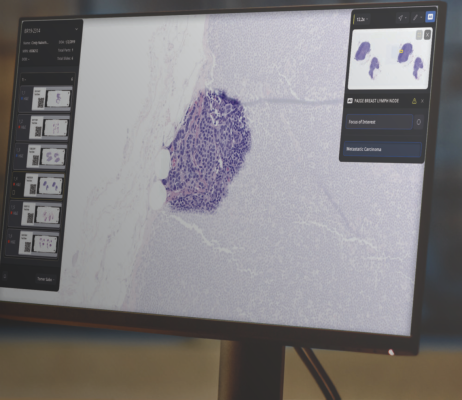
January 9, 2024 — Paige, a global leader in end-to-end digital pathology solutions and clinical AI applications, today announced the release of a groundbreaking product developed from Paige’s Pathology Foundation Model, Virchow. Built using one of the largest libraries of digitized images, utilizing unique computational resources provided by Microsoft Research, Paige has developed the first application that can detect cancer across more than 17 different tissue types including skin, lung, and the gastrointestinal tract, along with multiple rare tumor types and metastatic deposits.
Traditionally, development of pathology cancer detection AI applications required large datasets, one tissue type at a time, often taking months or years to build at clinical grade. By leveraging its unique Foundation Model, Paige has surpassed these limitations. With data derived from over 4 million digitized slides, Paige's innovative approach removes the constraints of developing single tissue products, making it possible to efficiently create cancer detection AI applications across a multitude of tumor types, a first in AI-based cancer diagnosis.
“The early success of our Foundation Model has been possible due to the size, quality, and diversity of the datasets we used to build it,” said Siqi Liu, PhD, director of AI Science at Paige. “Paige has access to one of the largest and most highly regarded pathology datasets globally, which allows us to leverage cutting-edge deep-learning approaches to train systems to detect common, complex, and even very rare cancer entities. Paige’s development provides the pathology community with the most powerful tools for diagnosis, prognosis, biomarker development, and targeted selection of patients for precision therapy,” he continued.
The exceptional performance of Paige’s multi-cancer application across various tissue types is considered state-of-the art in cancer pathology AI1. Paige is committed to ensuring its AI applications are clinical grade and will therefore continue to seek FDA regulatory oversight for products based on the Foundation Model technology.
“We see FDA clearance as being critical to ensure that regulatory and safety standards are being upheld in the application of AI in cancer diagnostics across tumor types,” said Andy Moye, CEO of Paige. “Paige remains at the forefront of innovation and regulatory milestones, and we expect this multi-tissue detection model to benefit patients, pathologists, and the broader medical community. Our commitment to excellence is exemplified by this groundbreaking achievement, marking a significant leap forward in cancer diagnostics.”
“Beyond its multi-tissue capabilities, the Foundation Model and the use of its embeddings can also be leveraged as a critical building block in a variety of upstream and downstream applications across the entire healthcare continuum,” said Razik Yousfi, Senior Vice President of Technology at Paige. “By combining the outputs of the Foundation Model with data types from other modalities, including genomics, radiology, and other health data, one can derive exponentially greater insights about the nature of cancer, its behavior, and response to specific treatments.”
For more information: https://paige.ai/
Reference:
1 https://arxiv.org/pdf/2309.07778.pdf Vorontsov E, Bozkurt A, Casson AI et al. VIRCHOW: A MILLION-SLIDE DIGITAL PATHOLOGY FOUNDATION MODEL. ArXiv. Preprint posted online September 14, 2023. arXiv:2309.07778v4


 May 17, 2024
May 17, 2024