November 18, 2020 — Ikonopedia announced the release of its newly updated next-generation breast MRI reporting module. The intuitive new interface is designed to reduce the complexity of reporting for screening and diagnostic MRI exams and is compliant to the ACR BI-RADS Atlas Fifth Edition.
The new breast MRI module leverages the intuitive icon-based interface of Ikonopedia's mammography and ultrasound structured reporting modalities to deliver a variety of physician efficiency and patient safety benefits. Reporting capabilities have been expanded and instinctual organization guides radiologists through BI-RADS criteria to reach an accurate, BI-RADS-compliant, and natural sounding description of lesions. New functionality in the MRI diagnostic modality includes ten lesion assessment categories that adhere to BI-RADS. The MRI screening modality has been updated to include a new contrast selection dialog as well as to synchronize with the new MRI diagnostic modality.
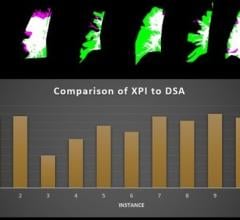
The enhanced breast MRI module has also been optimized for AI input such as Qlarity Imaging's QuantX, the first U.S. Food and Drug Administration (FDA)-cleared computer-aided diagnosis software for breast MRI analysis.
"We've been very pleased with the flexibility and efficiency gains from the intuitive user interface in the updated breast MRI reporting tools, particularly the ability to easily describe trackable entries while maintaining BI-RADS verbiage to create complex reports," said Erica Guzalo, Section Chief, Breast Imaging, Sinai Health Chicago. "I also appreciate Ikonopedia's dedication to continually help solve issues and implement new ideas that are beneficial to us, as users."
"As we, as an industry, move towards more broadly adopting risk-based screening based on a women's personal risk and breast density, the utilization of breast MRI will continue to grow," said Michael Vendrell, M.D., co-founder of Ikonopedia. "This new module streamlines reporting workflow to deliver more accurate diagnoses, reduces the risk of reporting errors, and save time as radiologists face increasing exam volume and data complexity. These are critical new capabilities to improve patient care and safety."
Ikonopedia is an innovative structured breast reporting and MQSA management system designed to dramatically improve reporting efficiency, and optimize facility operations. All findings are saved as discrete data which allows Ikonopedia to prevent errors, maintain BI-RADS-compliant language and automate many time-consuming processes. Ikonopedia makes it possible to eliminate laterality errors, automatically choose exam-appropriate patient letters and pull forward findings from past exams along with many other time-saving features.
Ikonopedia's integrated risk assessment tool is now available in dozens of languages and risk data is used to create alerts for the radiologist, populate the clinical section of the report, and automatically update the patient letter. A high-risk patient alert identifies patients with a 20% or greater lifetime risk and information about the score is instantly viewable.
For more information: www.ikonopedia.com


 May 31, 2024
May 31, 2024