Image courtesy of Aidoc
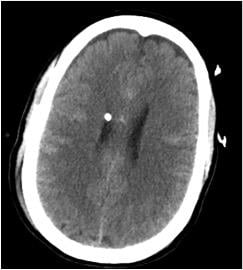
August 8, 2018 — Aidoc announced that it was granted U.S. Food and Drug Administration (FDA) clearance for the first product of its expanding suite of artificial intelligence (AI)-based workflow optimization solutions. The clearance is for Aidoc’s brain solution that works with radiologists to flag acute intracranial hemorrhage (ICH) cases in head computed tomography (CT) scans. Aidoc calls it the world's first deep learning solution to assist radiologists in workflow triage.
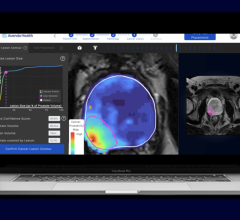
With over 75 percent of all patient care involving radiology, the amount of imaging is skyrocketing while the number of radiologists is struggling to catch up. Radiologists are being pressured to produce quality results at a faster pace with increasing amounts of data — but with tools that are not up to the task, according to Aidoc. The company’s solution analyzes medical images directly after the patient is scanned and notifies the radiologist of cases with suspected findings to assist with prioritization of time-sensitive and potentially life-threatening cases.
“In a recent clinical study we ran, Aidoc’s brain package demonstrated its potential to substantially reduce report turnaround time and increase the radiologist’s confidence” said Barry D. Pressman, M.D., chairman of imaging at Cedars-Sinai Medical Center and former president of the American College of Radiology. “Seeing the software in action emphasized the key aspects an AI solution needs to possess to have an impact on the radiologist day to day — seamless integration into the workflow and broad applicability. With the evidence I’ve seen, in the not so distant future, it will almost be unthinkable to practice radiology without the assistance of solutions like Aidoc”
Already commercialized outside the U.S. since December 2017, Aidoc is in the process of FDA clearance for its full body coverage solutions which will assist in the detection of a broad set of acute pathologies.
For more information: www.aidoc.com

 May 16, 2024
May 16, 2024