February 13, 2019 — At the 2019 Healthcare Information and Management Systems Society (HIMSS) global conference and exhibition, Feb. 11-15 in Orlando, Fla., Siemens Healthineers will demonstrate how healthcare providers can benefit from digitalization, particularly in the form of artificial intelligence (AI). In addition to its greater digital services portfolio, Siemens Healthineers will showcase two AI-based applications, the AI-Pathway Companion and the AI-Rad Companion.
The company will also present various digital solutions that have the potential to both help improve patient care and help reduce costs. These include several information technology (IT) tools for laboratory diagnostics, as well as the syngo Virtual Cockpit, a solution that enables medical professionals to connect remotely to scanner workplaces to assist personnel at a different location.
Siemens is also currently investigating AI-powered Digital Twin technology, which aims to enable the simulation of individual organ physiology to potentially better understand patient health and predict changes and therapy outcomes.
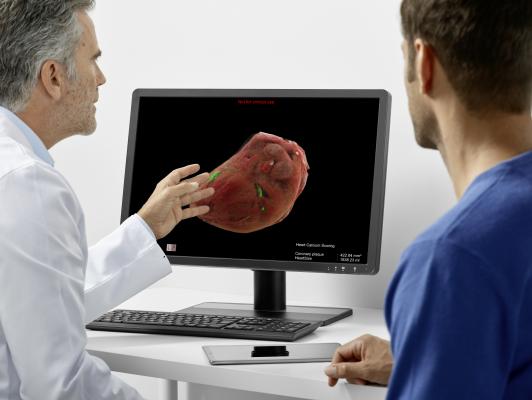
- The AI-Pathway Companion, which is currently under development, will be a clinical decision support system based on clinical guideline data imported by AI that can aid physicians in making diagnostic and therapeutic decisions along the clinical pathway. While numerous applications in the healthcare market make the workflows of individual clinical or administrative departments more efficient, the AI-Pathway Companion is designed to help optimize the processes along clinical pathways, and thus support personalized as well as standardized patient management. The design goal of the AI-Pathway Companion is to provide physicians in multi-disciplinary disease boards with the clinical status of each patient, based on data integration and artificial intelligence, and to help make recommendations for next steps to accelerate diagnostic and treatment decisions.
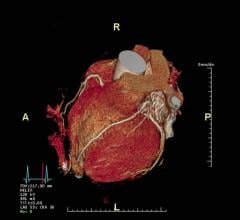
- The AI-Rad Companion Chest CT, which is pending U.S. Food and Drug Administration (FDA) 510(k) clearance, will be the first intelligent software assistant from Siemens Healthineers for radiology – and the first application of the AI-Rad Companion platform. The AI-Rad Companion Chest CT (computed tomography) is designed to identify anatomies and potentially disease-relevant changes, differentiate between various structures and highlight them individually, and mark and measure potential abnormalities. This applies to organs such as the lungs, heart, aorta and coronary arteries. The software is designed to automatically turn findings into a quantitative report.
- The syngo Virtual Cockpit software solution, which is not yet commercially available, can be used by medical staff to connect remotely to scanner workplaces to assist personnel at a different location, especially where more sophisticated examinations are required. syngo Virtual Cockpit can be utilized with magnetic resonance imaging (MRI) and MR-positron emission tomography (PET) scanners as well as with CT systems from Siemens Healthineers. With the ability to deploy experienced technologists across multiple locations, healthcare providers can achieve a higher level of standardization that can lead to more accurate diagnoses.
For more information: www.usa.healthcare.siemens.com


 April 24, 2024
April 24, 2024