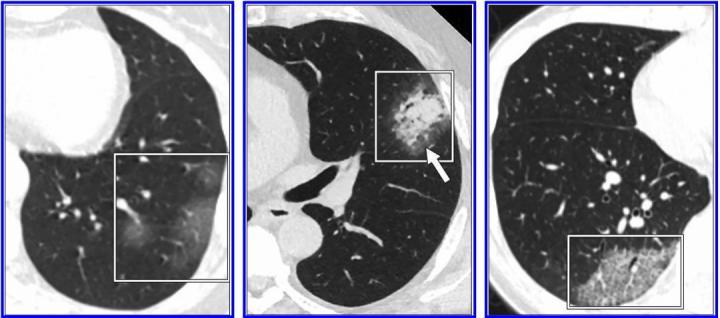
According to the 10 authors from multiple institutions across the US who reviewed the most frequently cited studies on the subject: 'Test performance and management issues arise when inappropriate and potentially overreaching conclusions regarding the diagnostic performance of CT for COVID-19 pneumonia are based on low-quality studies with biased cohorts, confounding variables, and faulty design characteristics. Image courtesy of American Journal of Roentgenology (AJR)
April 17, 2020 — To date, the radiology literature on coronavirus disease (COVID-19) pneumonia has consisted of limited retrospective studies that do not substantiate the use of computed tomography (CT) as a diagnostic test for COVID-19, according to an open-access Clinical Perspective article in the American Journal of Roentgenology (AJR).
"This is not to say these studies are not valuable," maintained lead investigator Constantine A. Raptis, M.D., of Washington University in Saint Louis. As Raptis, Travis S. Henry, M.D., of the University of California-San Francisco, and nine co-authors from six institutions across the United States noted of the most frequently cited studies on the subject, reporting the various CT features of COVID-19 pneumonia remains "an important first step" in helping radiologists identify patients who may have COVID-19 in the appropriate clinical environment.
"However," they continue, "test performance and management issues arise when inappropriate and potentially overreaching conclusions regarding the diagnostic performance of CT for COVID-19 pneumonia are based on low-quality studies with biased cohorts, confounding variables, and faulty design characteristics."
CT Sensitivity
Because misdiagnosing even a single patient (i.e., obtaining a false-negative finding) could result in large outbreaks among future contacts, understanding the potential effects of selection bias is important in determining sensitivity. As Raptis and colleagues explained, "if a study cohort contains patients who are more likely to have a true-positive finding and less likely to have a false-negative finding, sensitivity will be overestimated."
CT Specificity
The specificity and positive predictive value of a laboratory test — in the case of COVID-19, reverse transcription-polymerase chain reaction (RT-PCR) — are based on its ability to limit false-positive findings. Acknowledging false-positive RT-PCR results are possible, Raptis, Henry, et al. maintained they are often caused by contamination and are likely insignificant in the setting of assays for COVID-19. CT, on the other hand, does not test for singular features unique to the disease, and even those features most characteristic of COVID-19 pneumonia — peripheral, bilateral ground-glass opacities typically in the lower lobes — have been reported in a number of other conditions, both infectious and noninfectious.
CT in Clinical Practice
Finally, Raptis and colleagues addressed the hazards of wide deployment of CT: overuse of hospital resources, including the use of PPE already limited in availability but required to safely perform CT studies; clustering of affected and nonaffected patients in the radiology department, increasing the risk of disease transmission among imaging staff.
"At present," the authors of this AJR article concluded, "CT should be reserved for evaluation of complications of COVID-19 pneumonia or for assessment if alternative diagnoses are suspected."
Moderated by AJR Cardiopulmonary Imaging Section Editor Patrick M. Colletti, Constantine A. Raptis and Travis S. Henry served as speakers for a recent AJR Live Webinar exploring the radiography and CT findings associated with EVALI and COVID-19 — including the limitations of imaging in their diagnosis.
For more information: www.arrs.org


 April 23, 2024
April 23, 2024