May 8, 2014 — Cianna Medical announced the results of a trial examining the long-term side effects of strut-based brachytherapy for early-stage breast cancer. According to researchers, treatment with SAVI breast brachytherapy resulted in very low rates of late toxicities, even in patients with minimal space between the tumor cavity and skin.
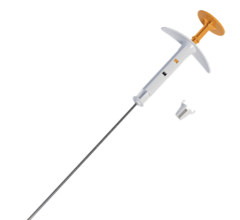
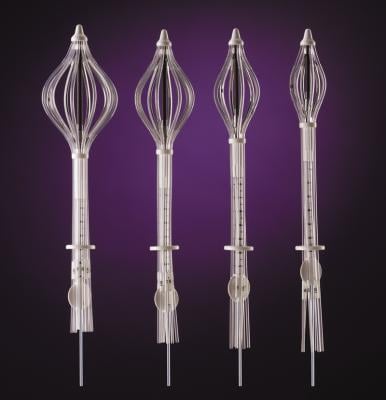
SAVI is a strut-based device that delivers a form of accelerated partial breast irradiation (APBI) known as breast brachytherapy, a five-day course of targeted radiation for early-stage breast cancer. With more than 10 years of clinical evidence showing excellent outcomes in efficacy and safety, breast brachytherapy is proven to be an appropriate treatment for early-stage breast cancer.
“The strut-based, open architecture design of SAVI allows physicians to safely deliver radiation, even in close proximity to the skin or chest wall, which offers a significant advantage over other brachytherapy techniques,” said study co-author Emily Penman, M.D., medical director of the Helen F. Graham Cancer Center at Christiana Care Health System, Newark, Del. “Our study confirms that strut-based brachytherapy allows us to treat any woman who is otherwise eligible for partial breast radiation with minimal risk of long-term toxicities.”
The study was presented at the annual meeting of the American Society of Breast Surgeons in Las Vegas. It examines 468 patients (469 breasts) treated with strut-based brachytherapy from 2007 to 2011. Of that group, 113 patients (114 breasts) had skin bridges of 5 mm or less. Late cutaneous toxicities (hyperpigmentation, erythema and telangiectasias) were analyzed for the entire cohort and those patients with small skin bridges.
Toxicity post-treatment for the entire group (with a median follow up of 27 months) was low: hyperpigmentation – 0.2 percent; erythema – 1.5 percent; and telangiectasias – 1.3 percent. Toxicities were similarly low for the ? 5 mm skin bridge group (which had a median follow up of 33 months): hyperpigmentation – 0.0 percent; erythema – 2.6 percent; and telangiectasias – 1.8 percent.
Data came from the SAVI Collaborative Research Group (SCRG), which studies the long-term outcomes of women treated with strut-based brachytherapy. Additional data from the SCRG has shown excellent local control with strut-based brachytherapy, with a 2 percent recurrence rate at four-and-a-half years median follow-up.
“Strut-based breast brachytherapy is proving to be a safe and effective way to deliver partial breast radiation,” said lead author Jon Strasser, a radiation oncologist at the Helen F. Graham Cancer Center. “This is great news for our patients, who are thrilled to have access to a treatment that delivers highly targeted radiation in a much more convenient way.”
Strut-based brachytherapy is used as part of breast conservation therapy, which consists of a lumpectomy followed by radiation therapy. Traditionally, the radiation portion consisted of whole breast irradiation (WBI), which requires six to seven weeks of daily treatments. Breast brachytherapy delivers radiation to the area immediately surrounding the lumpectomy cavity, sparing healthy tissue from unnecessary radiation and enabling treatment to be completed in as little as five days. Patient eligibility for breast brachytherapy is based on a number of factors, including tumor size, type of cancer, age, margin status and lymph node involvement.
For more information: www.ciannamedical.com


 October 01, 2023
October 01, 2023