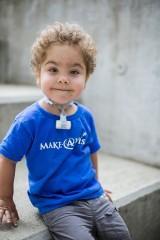
Kaibba Gionfriddo, one of the children suffering from TBM who was treated with a 3D-printed tracheal splint at C.S. Mott Children's Hospital at the University of Michigan
January 5, 2016 — In December, Materialise announced a partnership with Tissue Regeneration Systems (TRS) to manufacture 3D-printed tracheal splints for use in clinical trials Materialise will undertake with the University of Michigan in a separate exclusive licensing agreement. The company plans to ultimately offer the newly granted patent in the marketplace.
Materialise’s Mimics Innovation Suite was used to design the splint, which is constructed from a bioresorbable technology platform licensed to TRS by the University of Michigan in 2007. After several years refining fabrication methods, TRS received its first commercial product clearance from the U.S. Food and Drug Administration (FDA) in 2013.
Thanks to FDA approval for Expanded Access to an investigational medical device, the splints have already saved the lives of four infants suffering from tracheobronchomalacia (TBM), a life-threatening congenital airway disorder, since 2013.
About 1 in 2,200 babies are born with TBM, which causes the trachea to periodically collapse. The tracheal splint, developed to save the lives of these children, is made with a biopolymer called polycaprolactone, a biodegradable material that is gradually absorbed into the infant’s body tissue over time. Glenn Green, M.D., and Scott Hollister, Ph.D.,, of the University of Michigan used Materialise’s Mimics Innovation Suite to model and construct these splints using computed tomography (CT) scans of patient anatomy.
“This agreement is a critical step in our goal to make this treatment readily available for other children who suffer from this debilitating condition,” said Green.
“We have continued to evolve and automate the design process for the splints, allowing us to achieve in two days what used to take us up to five days to accomplish,” added Hollister, Ph.D., professor of biomedical engineering and mechanical engineering. “I feel incredibly privileged to be building products that surgeons can use to save lives.”
The U-M team hopes to next year open a clinical trial for 30 patients with similar conditions at C.S. Mott Children’s Hospital.
For more information: www.materialise.com


 February 01, 2024
February 01, 2024