GE Unveils Enhanced MRI Imaging Technology for Joint Replacements, Implanted Devices
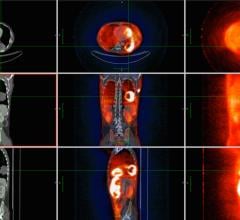
May 20, 2013 — At an event held at Hospital for Special Surgery, GE Healthcare introduced Mavric SL, a novel magnetic resonance (MR) imaging technique designed to address the growing clinical need to more accurately image soft tissue and bone in patients with MR conditional-labeled implants, such as joint replacements and other instrumentation. According to a Hospital for Special Surgery study published in The Journal of Bone & Joint Surgery, MR imaging can detect inflammation of the joint lining (synovitis) in patients with metal-on-metal hip implants long before symptoms appear, allowing for a more conclusive diagnosis and effective planning for follow-up care.
There are more than 1 million hip or knee replacement (arthroplasty) procedures performed each year in the United States[1] and more than 250,000 procedures are performed in Europe annually.[2] The need for arthroplasty revision procedures (a second surgery to correct the failure of an artificial joint) is accelerating significantly due to the increased frequency of joint replacements and the younger ages at which they are being performed. It is estimated that by 2030, the number of revision procedures will increase by 137 percent for hips and 601 percent for knees from 2005.[3]
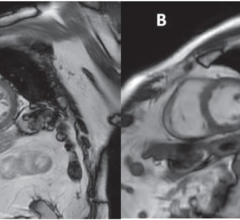
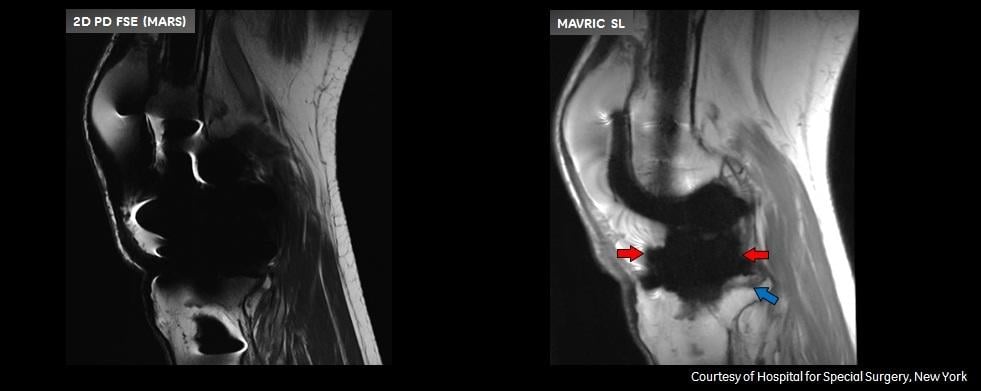
Patients with complications from joint replacement surgeries may present with pain and/or altered gait mechanics, or may have no symptoms at all. Prior to the availability of Mavric SL, achieving quality diagnostic MR images of the anatomy near implants was often not possible due to image distortion caused by metal used in implanted devices. Mavric SL reduces image distortion in the regions near MR conditional metal implants, enabling physicians to see tissue surrounding an implant to help them with diagnosis and defining a course of treatment. In some cases, Mavric SL can reduce the need for biopsy or exploratory surgery.
“The addition of Mavric SL to a standardized MR protocol is instrumental in providing accurate, reproducible diagnosis of adverse tissue reactions around implants,” said Hollis Potter, chief of MR imaging at Hospital for Special Surgery in New York and a lead member of the development team. “Even in asymptomatic patients, the Mavric SL technology can recognize an issue that needs to be monitored, providing valuable clinical information for an issue that can have significant human and economic costs, particularly when diagnosis is delayed.”
According to data published by Hospital for Special Surgery, patients may be experiencing tissue damage from metal-on-metal hip implants before pain symptoms appear. MR imaging was able to detect inflammation in both symptomatic and asymptomatic patients, helping to identify those patients who may need revision surgery before the surrounding tissue sustains further damage that makes revision more difficult. The study findings also underscore that commonly-used markers for tissue damage are insufficient in their ability to accurately identify those patients who may require revision surgery.[4]
“The development of the Mavric protocol has given us a window into the local tissue response to implants and a better understanding of the underlying cause of patients’ pain and poor function,” said Mathias P. Bostrom, M.D., attending orthopedic surgeon, Hospital for Special Surgery. “It is well established that there is more morbidity associated with revision in the setting of severe tissue loss, and Mavric SL positions clinicians to provide an early and accurate diagnosis.”
Mavric SL was developed as an innovative solution to a major clinical problem, uncovered as a result of a collaborative effort between GE, Hospital for Special Surgery and Stanford University. It is one of the latest offerings in GE’s MR portfolio designed to increase access to MR imaging for patients with metallic implants, all while maximizing patient care and reducing costs on the patient and healthcare system as a whole.
“GE Healthcare is committed to Humanizing MR by focusing on the needs of the patient, technologist and clinician,” said Richard Hausmann, president and CEO of GE Healthcare’s magnetic resonance business unit. “Current MR technology is limited and Mavric SL addresses this major gap in patient care, as the number of procedures requiring MR technologies continues to grow.”
Mavric SL is a combination of an acquisition technique and post-processing software intended for use on GE 1.5T and 3.0T MR systems. Mavric SL is suitable for use on all patients with passive MR conditional orthopedic implants that are scanned according to the conditions of safe use for the specific MR conditional implant being scanned. In addition, Mavric SL is suitable for use on patients without implants that are cleared for MR exams. Mavric SL helps reduce artifacts caused by presence of metal in both in-plane and through-plane dimensions compared to conventional MR imaging techniques. Thus Mavric SL allows visualizing more tissue in the vicinity of MR conditional implanted metal instrumentation. When interpreted by a trained physician, images generated by Mavric SL provide information that can be useful in determining a diagnosis. Mavric SL is non-invasive, radiation-free and needle-free as it requires no contrast media injection. Mavric SL received Food and Drug Administration (FDA) 501(k) clearance in December 2012 and is available in most markets.
For more information: www.gehealthcare.com
References:
1. Centers for Disease Control and Prevention/National Center for Health Statistics. "National Hospital Discharge Survey: 2010 table, Procedures by selected patient characteristics - Number by procedure category and age." Retrieved May 6, 2013, available at: http://www.cdc.gov/nchs/fastats/insurg.htm
2. Organisation for Economic Co-operation and Development (OECD). "Health at a Glance: Europe 2012" (Hip and Knee Replacement). Retrieved May 6, 2013, available at: http://www.oecd-ilibrary.org/social-issues-migration-health/health-at-a….
3. Kurtz S, Mowat F, Ong K, et al. "Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030." J Bone Joint Surg Am 2007;89:780–785.
4. Nawabi D, Potter HG, et al. "Magnetic Resonance Imaging Finding in Symptomatic versus Asymptomatic Subjects Following Metal-on-Metal Hip Resurfacing Arthroplasty." Journal of Bone & Joint Surgery. Vol 95, Issue 9.


 April 04, 2024
April 04, 2024